
A mosquito of the Aedes genus responsible for transmitting the Dengue virus to humans
La Dengue è tornata a far parlare di sé con un aumento di casi in diversi Paesi. Porto Rico è stata particolarmente colpita e nel marzo 2024 l’isola ha dichiarato la sua prima epidemia di Dengue dal 2012. In Europa ci sono tre Paesi in cui la Dengue è approdata e l’Italia è il Paese europeo con il maggior numero di casi.
Fortunatamente la Dengue non causa malattie gravi in tutte le persone che infetta, ma i sintomi possono essere spiacevoli e a volte richiedono un intervento medico.
Cliccate sulla galleria per imparare a conoscere i sintomi della Dengue.
Virus che nasce nelle zanzare

La febbre Dengue, altrimenti nota come Dengue, è un’infezione diffusa dalle zanzare. Tuttavia, non sempre provoca malattie gravi; infatti, solo una persona su quattro si ammala.
Dove è diffusa?
La Dengue è diffusa in alcune parti del mondo, tra cui alcune zone dell’Africa e dell’Asia, dell’America centrale e meridionale, dei Caraibi, delle isole del Pacifico e di alcune aree meridionali del Nord America.

È inoltre possibile contrarre la dengue in alcune zone dell’Europa meridionale in determinati periodi dell’anno (dalla primavera a Novembre).

Tra i Paesi in Europa in cui sono stati riscontrati casi di Dengue ci sono Croazia, Francia, Italia e Spagna.
Porto Rico

Sebbene la febbre dengue non sia presente ovunque nel mondo, di recente è stata al centro delle cronache per l’aumento dei casi, in particolare a Porto Rico.
Numeri
In effetti, Porto Rico ha dichiarato almeno 549 casi già nel 2024, rispetto a un numero totale di 1.293 casi per l’anno 2023.
Epidemia
Secondo il dipartimento sanitario dell’isola, più di 340 persone sono state ricoverate in ospedale a causa del virus e il Paese ha dichiarato l’epidemia.
Rischio pandemico

È la prima volta che Porto Rico dichiara un’epidemia di Dengue dal 2012, e ciò avviene dopo che l’Organizzazione Mondiale della Sanità (OMS) ha avvertito che la Dengue è un rischio pandemico nel Gennaio 2024.
Modello più ampio

In effetti, l’epidemia di Porto Rico fa parte di un modello più ampio che è emerso in tutte le Americhe fino ad ora nel 2024.
Altri Paesi

Paesi come Argentina, Brasile, Perù e Uruguay hanno riportato un numero significativo di casi di Dengue.
Riconoscere i sintomi

Anche se l’infezione da Dengue non sempre provoca la malattia, è comunque una buona idea saper riconoscere i sintomi.
Viaggiare verso un Paese dove c’è la Dengue

Questo è particolarmente vero se si intende viaggiare in un Paese in cui il numero di casi di dengue è in aumento.
Quanto ci mettono i sintomi a manifestarsi?

Se i sintomi della Dengue si manifestano, di solito si sviluppano nei 4-10 giorni successivi alla puntura di una zanzara infetta.
Sintomi simili a quelli influenzali

La febbre Dengue può sembrare un’influenza e i sintomi sono simili. Essi comprendono una temperatura elevata, un forte mal di testa e dolore dietro gli occhi.
Altri sintomi
Altri sintomi includono dolori muscolari e articolari, sensazione di malessere, ghiandole gonfie e un’eruzione cutanea costituita da macchie piatte o leggermente rialzate.
Quando consultare un medico

In generale, le persone che sviluppano i sintomi della Dengue si sentono meglio dopo circa una settimana. Tuttavia, è importante rivolgersi a un medico se si è viaggiato di recente in un paese in cui è presente la Dengue e si manifestano i sintomi.
Casi di Dengue più gravi

Questo è importante perché alcune persone sviluppano un tipo di Dengue più grave pochi giorni dopo aver iniziato a sentirsi male. Si tratta di un’eventualità rara, ma non sconosciuta.
Popolazione a rischio

Chi ha già avuto la Dengue in passato ha maggiori probabilità di sviluppare una Dengue grave. È anche più comune nelle donne in gravidanza e nei neonati.
Sviluppare la Dengue con sintomi gravi

Le persone che sviluppano una Dengue grave possono iniziare a sentirsi meglio e vedere la loro temperatura tornare alla normalità, solo per sviluppare sintomi ancora più gravi 24-48 ore dopo.
Sintomi

I sintomi della Dengue grave comprendono forti dolori alla pancia, vomito ripetuto, respirazione accelerata, sanguinamento delle gengive o del naso, affaticamento, irrequietezza e vomito o feci sanguinolente.
Terapia ospedaliera

La dengue grave può diventare molto seria se non viene trattata adeguatamente in ospedale. È quindi importante rimanere vigili e rivolgersi a un medico quando necessario.
Trattamenti

Non esiste un trattamento specifico per la Dengue, ma è possibile alleviare i sintomi riposando, bevendo molti liquidi e assumendo paracetamolo.
Non assumere ibuprofene o aspirina

È importante, tuttavia, non assumere antidolorifici antinfiammatori, come l’ibuprofene o l’aspirina. Questi possono causare problemi di sanguinamento nelle persone affette da Dengue.
Evitare i viaggi

Se si appartiene a uno di questi gruppi, è consigliabile evitare di viaggiare nei Paesi in cui è presente questa infezione.
Evitare le punture di zanzara

Per le persone che viaggiano in Paesi in cui è presente la Dengue, il modo migliore per prevenire l’infezione è evitare di essere punti dalle zanzare.
Proteggersi con i vestiti

È buona norma indossare indumenti a maniche lunghe e pantaloni per coprire braccia e gambe, soprattutto nelle prime ore del mattino e della sera, quando le zanzare sono più numerose.
Repellente per insetti

Si può anche usare un repellente per insetti sulla pelle, preferibilmente uno che contenga DEET, e si dovrebbe cercare di chiudere le finestre e le persiane quando possibile.
Zanzariera

Infine, è buona norma dormire sotto una zanzariera trattata con insetticida, anche quando si dorme di giorno.
In sintesi

La febbre Dengue può essere fastidiosa e i casi sono in aumento. Anche se è raro ammalarsi di questa infezione, vale la pena di rimanere vigili e di essere consapevoli dei sintomi.
Fonti: (NHS) (CDC)

Febbre Dengue
Informazioni generali
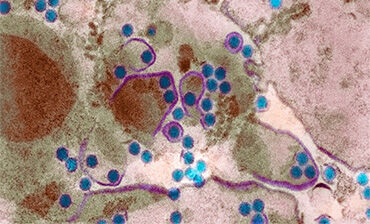
Di origine virale, la dengue è causata da quattro virus molto simili (Den-1, Den-2, Den-3 e Den-4) ed è trasmessa agli esseri umani dalle punture di zanzare che hanno, a loro volta, punto una persona infetta. Non si ha quindi contagio diretto tra esseri umani, anche se l’uomo è il principale ospite del virus. Il virus circola nel sangue della persona infetta per 2-7 giorni, e in questo periodo la zanzara può prelevarlo e trasmetterlo ad altri.
Nell’emisfero occidentale il vettore principale è la zanzara Aedes aegypti, anche se si sono registrati casi trasmessi da Aedes albopictus. La dengue è conosciuta da oltre due secoli, ed è particolarmente presente durante e dopo la stagione delle piogge nelle zone tropicali e subtropicali di Africa, Sudest asiatico e Cina, India, Medioriente, America latina e centrale, Australia e diverse zone del Pacifico. Negli ultimi decenni, la diffusione della dengue è aumentata in molte regioni tropicali. Nei paesi dell’emisfero nord, in particolare in Europa, costituisce un pericolo in un’ottica di salute globale, dato che si manifesta soprattutto come malattia di importazione, il cui incremento è dovuto all’aumentata frequenza di spostamenti di merci e di persone.
Normalmente la malattia dà luogo a febbre nell’arco di 5-6 giorni dalla puntura di zanzara, con temperature anche molto elevate. La febbre è accompagnata da mal di testa acuti, dolori attorno e dietro agli occhi, forti dolori muscolari e alle articolazioni, nausea e vomito, irritazioni della pelle che possono apparire sulla maggior parte del corpo dopo 3-4 giorni dall’insorgenza della febbre. I sintomi tipici sono spesso assenti nei bambini.
Sintomi e diagnosi
La diagnosi è normalmente effettuata in base ai sintomi, ma può essere più accurata con la ricerca del virus o di anticorpi specifici in campioni di sangue.
Prevenzione e trattamento
La misura preventiva più efficace contro la dengue consiste nell’evitare di entrare in contatto con le zanzare vettore del virus. Diventano quindi prioritarie pratiche come l’uso di repellenti, vestiti adeguati e protettivi, zanzariere e tende. Dato che le zanzare sono più attive nelle prime ore del mattino, è particolarmente importante utilizzare le protezioni in questa parte della giornata.
Per ridurre il rischio di epidemie di Dengue, il mezzo più efficace è la lotta sistematica e continuativa alla zanzara che funge da vettore della malattia. Ciò significa eliminare tutti i ristagni d’acqua in prossimità delle zone abitate, ed effettuare vere e proprie campagne di disinfestazione che riducano la popolazione di Aedes.
A febbraio 2023, l’Agenzia Italiana del Farmaco (AIFA) ha autorizzato l’utilizzo e la commercializzazione di Qdenga (Takeda), un vaccino tetravalente vivo attenuato per la prevenzione della malattia da Dengue causata da uno qualsiasi dei quattro sierotipi del virus. Il vaccino ha ricevuto anche l’approvazione da parte dell’EMA (European Medicines Agency) a dicembre 2022. Un secondo vaccino il Dengvaxia (Sanofi Pasteur), non commercializzato in Italia, è indicato solo per persone residenti in aree endemiche e che abbiano avuto una precedente infezione da Dengue, confermata attraverso dei test di laboratorio.
Non esiste un trattamento specifico per la dengue, e nella maggior parte dei casi le persone guariscono completamente in due settimane. Le cure di supporto alla guarigione consistono in riposo assoluto, uso di farmaci per abbassare la febbre e somministrazione di liquidi al malato per combattere la disidratazione. In qualche caso, stanchezza e depressione possono permanere anche per alcune settimane.
La malattia può svilupparsi sotto forma di febbre emorragica con emorragie gravi da diverse parti del corpo che possono causare veri e propri collassi e, in casi rari, risultare fatali.
Fonte: Istituto Superiore di Sanità, Italia https://www.epicentro.iss.it/febbre-dengue/
English translate
Dengue Fever
General informations
Of viral origin, dengue is caused by four very similar viruses (Den-1, Den-2, Den-3 and Den-4) and is transmitted to humans by mosquito bites which, in turn, bite a person infected. There is therefore no direct contagion between humans, even if humans are the main host of the virus. The virus circulates in the blood of the infected person for 2-7 days, and in this period the mosquito can pick it up and transmit it to others.
In the Western Hemisphere the main vector is the Aedes aegypti mosquito, although cases transmitted by Aedes albopictus have been recorded. Dengue has been known for over two centuries, and is particularly present during and after the rainy season in the tropical and subtropical areas of Africa, Southeast Asia and China, India, the Middle East, Latin and Central America, Australia and several areas of the Pacific. In recent decades, the spread of dengue has increased in many tropical regions. In the countries of the northern hemisphere, particularly in Europe, it constitutes a danger from a global health perspective, given that it manifests itself above all as an imported disease, the increase of which is due to the increased frequency of movement of goods and people.
Symptoms and diagnosis
Normally the disease gives rise to fever within 5-6 days of the mosquito bite, with even very high temperatures. Fever is accompanied by sharp headaches, pain around and behind the eyes, severe muscle and joint pain, nausea and vomiting, skin irritations that may appear on most of the body 3-4 days after the onset of fever. Typical symptoms are often absent in children.
Diagnosis is normally made based on symptoms, but can be more accurate by looking for the virus or specific antibodies in blood samples.
Prevention and treatment
The most effective preventive measure against dengue is to avoid coming into contact with the mosquitoes that carry the virus. Practices such as the use of repellents, adequate and protective clothing, mosquito nets and curtains therefore become priorities. Since mosquitoes are most active in the early hours of the morning, it is especially important to use protection during this part of the day.
To reduce the risk of dengue epidemics, the most effective means is the systematic and continuous fight against the mosquito that acts as a vector of the disease. This means eliminating all stagnant water near inhabited areas, and carrying out actual disinfestation campaigns that reduce the Aedes population.
In February 2023, the Italian Medicines Agency (AIFA) authorized the use and marketing of Qdenga (Takeda), a live attenuated tetravalent vaccine for the prevention of Dengue disease caused by any of the four serotypes of the virus. The vaccine also received approval from the EMA (European Medicines Agency) in December 2022. A second vaccine, Dengvaxia (Sanofi Pasteur), not marketed in Italy, is indicated only for people residing in endemic areas and who have had a previous Dengue infection, confirmed through laboratory tests.
There is no specific treatment for dengue, and in most cases people recover completely within two weeks. Treatments to support recovery consist of absolute rest, use of drugs to reduce fever and administration of fluids to the patient to combat dehydration. In some cases, tiredness and depression can persist for a few weeks.
The disease can develop in the form of hemorrhagic fever with severe bleeding from different parts of the body which can cause real collapse and, in rare cases, be fatal.
Source: Istituto Superiore della Sanità (ISS) Italia
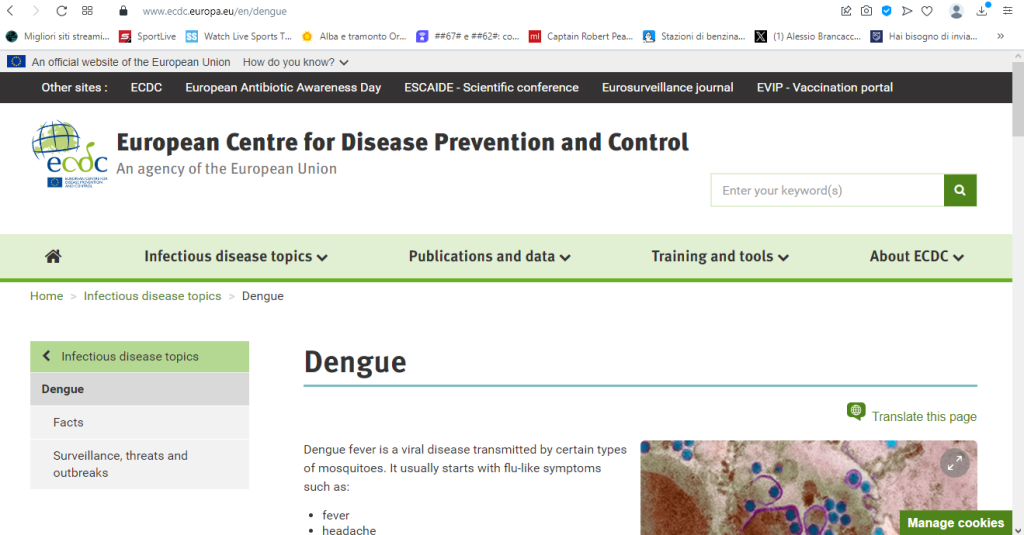
Dengue
Dengue fever is a viral disease transmitted by certain types of mosquitoes. It usually starts with flu-like symptoms such as:
- fever
- headache
- muscle and joint pain
- rash
Symptoms appear in humans 3-14 days after infection.
In some cases, the disease can become severe, leading to conditions like dengue hemorrhagic fever and dengue shock syndrome. When the disease is severe, the risk of mortality is higher. There are four types of viruses that cause dengue, and being immune to one type does not protect against the others
Key facts
Risk for people
Dengue outbreaks are sometimes seen in southern Europe and consequently it is closely monitored in the region..
Around the world, dengue is the most common viral disease transmitted by mosquitoes that affects people. Every year, there are tens of millions of cases are reported, and it causes about 20 000 to 25 000 deaths, with a higher impact on children.
How it spreads
Dengue is a disease caused by a virus that mainly spreads through mosquito bites. Mosquitoes get the virus by biting infected people and can transmit it to others when they bite again.
Vaccination and treatment
There is no specific treatment for dengue. Early diagnosis is crucial to enable the provision of appropriate supportive care to patients and to apply disease control measures in the area.
The are two vaccines against dengue; both s are primarily designed for use in areas where dengue is very common (i.e. not mainland Europe).
Protective measures
For individuals, protective measures include:
- using mosquito repellent
- the use of mosquito nets
- sleeping or in screened or air-conditioned rooms
- wearing clothing that covers most of the body.
Preventative measures also focus on controlling the mosquitoes that spread the virus.
Some ways to reduce mosquito breeding sites include:
- Regularly removing or treating open containers with stagnant water, like flower pots, tires, tree holes, and rock pools.
- Ensuring water containers, barrels, wells, and storage tanks are well covered.
During outbreaks, aerial spraying of insecticides can be used to get rid of adult mosquitoes and mitigate the spread of the disease.
Aedes aegypti (Yellow Fever Mosquito) – Factsheet for experts
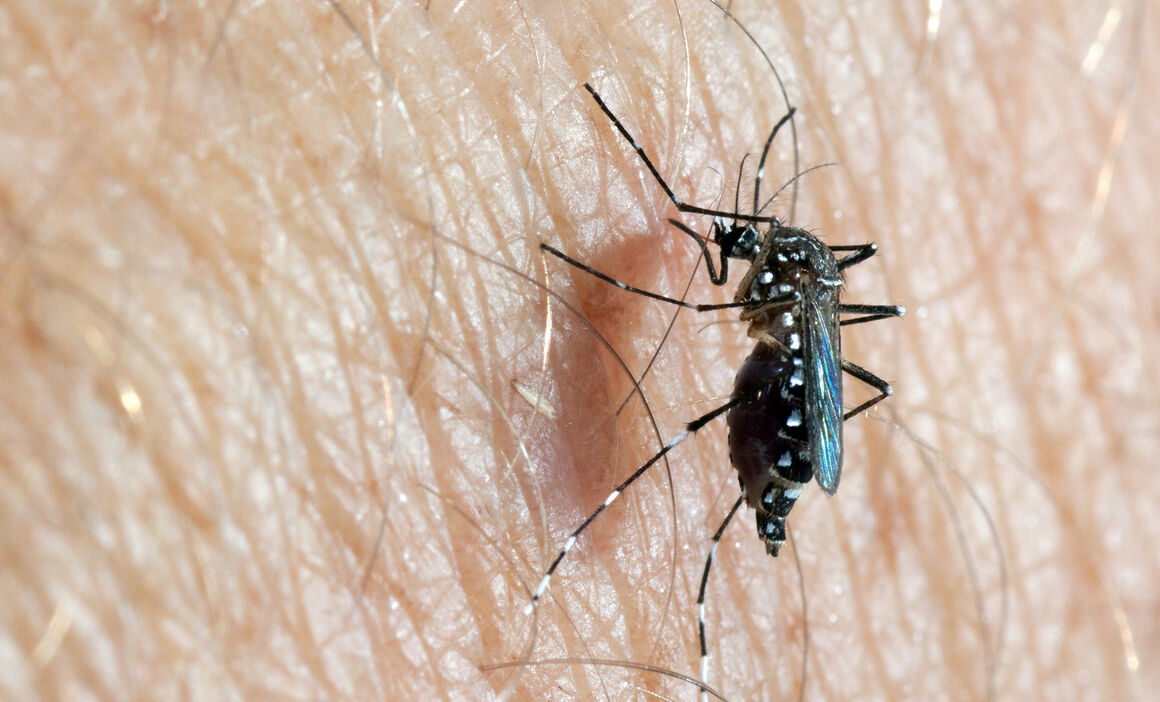
Species name/classification: Aedes (Stegomyia) aegypti
Common name: Yellow fever mosquito
Synonyms and other name in use: Stegomyia aegypti
Aedes aegypti is a known vector of several viruses including yellow fever virus, dengue virus, chikungunya virus, and Zika virus.
Index:
Hazards associated with mosquito species
Epidemiology and transmission of pathogens
Public health (control/interventions)
Hazards associated with mosquito species
Current issues
Invasive species
The invasive success of Ae. aegypti has largely been due to international travel and trade. Historically, Ae. aegypti has moved from continent to continent via ships and was previously established in southern Europe from the late 18th to the mid-20th century. Its disappearance from the Mediterranean, Black Sea and Macaronesian biogeographical region (Canary Islands, Madeira and the Azores) is not well understood [1,2]. It has since recolonised Madeira [3], reappeared in parts of southern Russia and Georgia (Krasnodar Krai and Abkhazia) [4], and reportedly been introduced into the Netherlands [5], Canary Islands [6,7] and Cyprus [8]. VectorNet field studies have shown the species to be widespread across extended areas of Georgia, including the capital city, Tbilisi, and it has also spread into north-eastern Türkiye [9]. Nowadays it is one of the most widespread mosquito species globally. If Ae. aegypti is introduced into southern Europe, there are no climatic or environmental reasons as to why it could not survive [10,11]. Dispersal via shipping (ferries) from Madeira is still thought to represent the greatest risk for the introduction of this mosquito into Europe. Although its global establishment is currently restricted due to its intolerance to temperate winters [13], over the past 30 years there has been an increase in its distribution worldwide [14].
Ecological plasticity
Ae. aegypti thrives in densely populated areas without reliable water supplies, waste management and sanitation [15]. It is suggested that Ae. aegypti evolved its domestic behaviour in West Africa, and its widespread colonisation and distribution across the tropics led to highly efficient inter-human transmission of viruses, such as dengue [16]. This domestic behaviour can provide protection from adverse environmental conditions (as it rests indoors) and offer numerous habitats suitable as oviposition sites, but also makes it vulnerable to (indoor) vector control measures [14].
Biting and disease risk
Aedes aegypti is a known vector of several viruses including yellow fever virus, dengue virus chikungunya virus and Zika virus. In Europe, imported cases infected with these viruses are reported every year [17,18]. Therefore, the potential establishment of this mosquito in Europe raises concerns about autochthonous transmission of these arboviruses [1-3,9,12,19], particularly in southern Europe where climatic conditions are most suitable for the re-establishment of the species. In 2012, a large outbreak of dengue fever, associated with Ae. aegypti, occurred in the Portuguese Autonomous Region of Madeira (on the African tectonic plate) [20]. The epidemic started in October 2012 and by early January 2013 more than 2 200 cases of dengue fever had been reported, with an additional 78 cases reported among European travellers returning from the island [21].
Geographical distribution
Historically Ae. aegypti has been reported as established in:
- all Mediterranean countries (Europe, Middle East and North Africa)
- in the Caucasus (southern Russia, Georgia, Azerbaijan)
- continental Portugal
- both the Atlantic archipelagos (Canaries and Azores) [2,22].
It is currently distributed throughout the tropics, including Africa (from where it originates) and a number of sub-tropical regions such as:
- south-eastern United States
- the Middle East
- South-East Asia
- the Pacific and Indian Islands
- northern Australia [23].
Although historically present in Europe, its current distribution is limited, but extending. The current known distribution of Ae. aegypti in Europe is displayed on the vector maps.
Brief history of spread and European distribution
Pathways
Aedes aegypti was most probably transported into the Americas and the Mediterranean on ships sailing from Africa [1,16,24]. The northernmost documented occurrences in Europe (Bordeaux and Saint Nazaire, France; Swansea and Southampton, UK) clearly result from introductions via ships, and there is no evidence that the species has become established in these places [2]. In the past, the species has been sporadically reported in Europe, from the Portuguese Atlantic coast to the Black Sea [2], displaying a much larger distribution than at present. The same also applies to North America and Australia [14]. The reduction in distribution is possibly due to elimination programmes.
Initial importations and spread in Europe
Aedes aegypti disappeared from Europe during the first half of the twentieth century (the species was reported in Spain up to 1953 and in Portugal up to 1956). Despite a few subsequent sporadic recordings (northern Italy, 1972; Israel, 1974; Turkey, 1961, 1984, 1992, 1993, 2001), it is only more recently that reports of re-colonisation have come to light [2]. Colonisation on the island of Madeira was reported as having started in 2004, and there are concerns that Aedes aegypti could be transported to western Europe via air or sea traffic [3]. Similarly, there are concerns that the species could be introduced into other countries bordering the Black Sea from Russia and Georgia via sea or road traffic, as this has already been shown to be the case in north-eastern Türkiye [9]. From there, the species could easily spread via road traffic to other parts of Türkiye, including Istanbul, and on to neighbouring EU states. Furthermore, Ae. aegypti has been reported to have been found in the Netherlands at tyre yards, undoubtedly imported via shipments of tyres originating from Florida, USA [5,25]. However, the control measures that were immediately applied have successfully eliminated the species from these foci.
Possible future expansion
Unlike Ae. albopictus, the ability of Ae. aegypti to establish itself in more temperate regions is currently restricted, due to its intolerance of temperate winters and, in particular, the high mortality rate of eggs when exposed to frost [13,26]. However, there is no reason why it should not become re-established widely across the Mediterranean. Coastal regions of the Mediterranean, the Black Sea, and the Caspian Sea, and areas along large lowland rivers (Ebro, Garonne, Rhone, and Po) have been identified as suitable habitats for Ae. aegypti [10]. Moreover, this could change in the future, with global climate change resulting in the species’ ability to expand further to the north and south [16]. Back to Top
Entomology
Species name/classification: Aedes (Stegomyia) aegypti (Linnaeus, 1762) [27]
Common name: Yellow fever mosquito
Synonyms and other name in use: Stegomyia aegypti (sensu Reinert et al., 2004) [28]
Morphological characters and similar species
Adults of Ae. aegypti are relatively small and have a black and white pattern due to the presence of white/silver scale patches against a black background on the legs and other parts of the body. Some indigenous mosquitoes also show such contrasts (more brownish and yellowish) but these are less obvious. However, Ae. Aegypti could be confused with other invasive (Ae. Albopictus, Ae. Japonicus) or indigenous species (Ae. Cretinus, restricted to Cyprus, Greece and Türkiye). The prevailing diagnostic character is the presence of silver scales in the shape of a lyre against a black background on the scutum (dorsal part of the thorax). The domestic form (Ae. Aegypti aegypti) is paler than its ancestor (Ae. Aegypti formosus) and has white scales on the first abdominal tergite. The latter is confined to Africa, south of the Sahara, and has been recorded as breeding in natural habitats in areas of forest or bush, away from places of human settlement [29].
Seasonal abundance
On the island of Madeira Ae. aegypti is active throughout the year, with a peak in abundance from August to October [30].
Voltinism (generations per season)
Multivoltine
Host preferences (e.g. birds, mammals, humans)
Aedes aegypti feeds on mammalian hosts [31], preferably humans, even in the presence of alternative hosts [32]. It also feeds multiple times during one gonotrophic cycle (feeding, egg-producing cycle) [14,16,33] which has implications for disease transmission.
Aquatic/terrestrial habitats
Historically, Ae. aegypti was found in forested areas, using tree holes as habitats [16]. As an adaptation to urban domestic habitats, nowadays it exploits a wide range of artificial containers such as vases, water tanks and tyres [14]. It also uses underground aquatic habitats, such as septic tanks [34], and can adapt to use both indoor and outdoor aquatic container habitats in the same area. Adaptation to breeding outdoors may result in increased population numbers and difficulty in implementing control methods [32]. A study in Brazil found high numbers of eggs in oviposition sites close to human populations [35]. Eggs which are laid on or near the water surface [14] are normally resistant to desiccation [36].
Biting/resting habits
The domestic form of Ae. aegypti is often found as close as 100 metres to human habitations [1] although some studies have shown that breeding habitats can also be found away from human dwellings [32]. Aedes aegypti prefer human habitations as they provide resting and host-seeking possibilities [16] and, as a result, they will readily enter buildings [1,14]. The activity of the species is both diurnal and crepuscular [14,31].
Environmental thresholds/constraints/development criteria
Aedes aegypti, unlike Ae. albopictus, is not able to undergo winter diapause as eggs, and this therefore limits its ability to exploit more northerly temperate regions (although some survival is possible during the summer following an importation). However, it may establish itself in regions of Europe with a humid sub-tropical climate (e.g. parts of the Mediterranean and countries around the Black Sea), such as the Sochi region where it has become re-established since 2001 [37]. Species competition has also been shown to affect distribution and abundance. A decrease in the distribution of Ae. aegypti has been associated with the invasion of Ae. albopictus, especially in south-eastern USA [14].
Aedes aegypti also has limited dispersal capability in its adult form [14], with a flight range estimated to be only 200 metres [31]. Rainfall may affect abundance and productivity of breeding sites but this species’ preference for artificial water containers means it does not have to rely on rainfall for the availability of larval development sites [14]. These aspects, coupled with its preference for feeding and resting indoors, make the species less susceptible to the effects of climatic factors, which could influence its distribution.
Epidemiology and transmission of pathogens
Known vector status
Aedes aegypti is known to transmit dengue virus, yellow fever virus, chikungunya virus, and Zika virus. It has been suggested as a potential vector of Venezuelan Equine Encephalitis virus [38] and vector competency* studies have shown that Ae. aegypti is capable of transmitting West Nile virus. West Nile virus has also been isolated from this mosquito species in the field [31].
Chikungunya
Aedes aegypti is the primary vector of chikungunya virus [39]. Transovarial transmission was demonstrated by Aitken et al. [40] under laboratory conditions and the virus has been detected in wild-caught male Ae. aegypti [41]. Transovarial transmission may help with the maintenance of the virus in nature [42]. Venereal transmission during mating has also been demonstrated under laboratory conditions, although it is thought to be less widespread than transovarial transmission [42].
Aedes aegypti has been involved in virtually all chikungunya epidemics in Africa, India and other countries in South-East Asia [42][43]. The species caused an outbreak of chikungunya in Kenya (2004) and the Comoros islands (2005), affecting 63% of the population in the latter case [44]. An entomological investigation following an outbreak of chikungunya virus in Yemen (2010/2011) revealed the presence of the virus in field-collected Ae. aegypti in the outbreak area [45]. More recently, Ae. aegypti was involved in large chikungunya outbreaks in the Pacific and the Caribbean [19,46,47]. As a consequence, Europe’s vulnerability to the virus has increased [17,19].
More information on the disease can be found on the fact sheet about chikungunya.
Dengue
Aedes aegypti is the primary vector of dengue [48]. All four dengue serotypes have been isolated from field-collected Ae. aegypti [49]. Vertical transmission of dengue virus types 2, 3 and 4 has been demonstrated [29] and although some suggest this is inefficient [49], others suggest that it plays a significant role in viral maintenance [50].
Aedes aegypti has long been recognised as a vector of dengue, causing major dengue fever epidemics in the Americas and South-East Asia. The global incidence of dengue has also increased over the past 25 years [14,51]. Historically, outbreaks have also been reported in Europe, with one of the largest outbreaks on record occurring in Athens and neighbouring areas of Greece during the period 1927–1928 [52] [2]. In 2012, a large outbreak of dengue fever occurred in the Portuguese Autonomous Region of Madeira [20] where Ae. aegypti is established.
More information on the disease can be found on the fact sheet about dengue.
Yellow fever
Yellow fever is maintained in a sylvatic cycle between monkeys and mosquitoes of Aedes or Haemagogus genera [29,53]. Aedes aegypti is the vector involved in urban transmission of yellow fever where only humans are the amplifying host. Aedes aegypti has been shown to transmit yellow fever virus transovarially to F1 progeny under laboratory conditions [40] and field collection studies have also confirmed this in nature [29].
Yellow fever transmission has been reported from countries across sub-Saharan Africa and in tropical areas across South and Central America, from Panama to the northern part of Argentina [54]. Autochthonous transmission of yellow fever has never been detected in Asia, although the Ae. aegypti vector is present in south and south-eastern areas of the continent [55].
More information on the disease can be found on the fact sheet about yellow fever.
Zika virus
Zika virus is maintained in a sylvatic cycle involving non-human primates and a wide variety of sylvatic and peri-domestic Aedes mosquitoes. Aedes aegypti is considered the most important vector for Zika virus transmission to humans. Aedes aegypti mosquitoes were found infected in the wild (reviewed in [56]). More recently, the species was found infected during the Zika virus outbreak in Brazil [57]. The mosquito has been shown to transmit the virus under laboratory conditions but differences in vector competence* between studies were reported [58-60].
More information on the disease can be found on the fact sheet Zika virus infection
Factors driving/impacting on transmission cycles
The spread of Ae. aegypti-borne diseases has been aided by the global spread of Ae. aegypti over the past 25 years [14]. Although currently limited in spread due to its intolerance to temperate winters, climate change could result in an increased distribution of Ae. aegypti.
As the human population grows, sites in which this mosquito can thrive will increase, providing further habitats. This fact, coupled with the close proximity of humans and the tendency of Ae. aegypti to feed on multiple hosts during one gonotrophic cycle [16][14][33], increases the risk of disease transmission in such areas. The movement of viraemic hosts can result in outbreaks from a number of arboviruses in non-endemic areas.
The re-establishment of Ae. aegypti in some areas has resulted in disease transmission. Inadequate control of this invasive species could lead to its re-establishment in Europe which is why surveillance and research on this mosquito is so important.
Public health (control/interventions)
Vector surveillance
Methods for surveying Ae. aegypti are addressed in ECDC’s ‘Guidelines for the surveillance of invasive mosquitoes in Europe’ [61].
ECDC and the European Food Safety Authority (EFSA) fund European-wide monitoring and mapping activities for invasive mosquito species and potential mosquito vectors (VectorNet).
Species specific control methods
Source reduction and adult control
Aedes aegypti thrives in urban environments which provide it with numerous oviposition sites to lay eggs. Therefore, the distribution of this species is largely driven by human activities (e.g. storage of water outside) and this should be the focus of control methods [14]. This is challenging because of the numerous sites in which Ae. aegypti lay eggs and in an urban setting, such sites are hard to access. For example, a study in Mexico used a combination of quadrat and transect sampling methods to identify the most important containers for pupal development in 600 houses. They found an association between Ae. aegypti pupae and large cement washbasins. Source reduction and targeted treatment of such sites could ensure that the use of insecticides is more successful in reducing mosquito numbers [62].
Historically, outbreaks of dengue and yellow fever have been controlled by Ae. aegypti eradication programmes but these have not always been successful and abandoning efforts led to the re-emergence of the diseases associated with this mosquito [63]. In the twentieth century, many eradication programmes were targeted at larval development sites in an attempt to eliminate yellow fever transmission. The use of DDT after the Second World War resulted in the eradication of the species from 22 countries in the Americas [1]. This effort was discontinued and Ae. aegypti quickly re-colonised nearly all of the neo-tropics and sub-tropics [16]. Since Ae. aegypti has become less accessible, due to the fact that the species spends more time indoors, outdoor insecticidal spraying has become less efficient [1]. Eradication programmes set up during the 1950–60s (initiated by the Pan American Health Organization) in the Americas saw the reduction and eradication of Ae. aegypti there, but relaxation of mosquito management after the 1970s resulted in the re-establishment of Ae. aegypti, followed by dengue outbreaks [14].
Some other methods used include the introduction of predators into the larval habitats of Ae. aegypti (e.g. copepods), the introduction of irradiated or genetically-modified mosquitoes (sterile male release) and the use of Wolbachia bacteria which can inhibit the replication of dengue virus within Ae. aegypti, thereby suppressing or eliminating dengue transmission [14]. Protective clothing and repellents are also advocated to reduce exposure to Ae. aegypti, as well as the spraying of indoor living spaces with pyrethrin [53]. Personal protective measures to reduce the risk of mosquito bites also include using mosquito bed nets (preferably insecticide-treated nets), and sleeping or resting in screened or air-conditioned rooms.
Integrated control programme
Implementation of an integrated control strategy against invasive mosquito species should take into account the target species, its ecology and the public health concern (i.e. nuisance and/or disease transmission). As a general rule, an integrated control strategy requires the coordinated involvement of local authorities, private partners, organised society and communities [64].
Traditional methods such as source reduction, public education and insecticide application are routinely implemented by municipalities to reduce Aedes populations, but with limited success, probably because of poor participation of communities, and a lack of coordination and synchronised implementation [64]. Innovative approaches, such as pyriproxyfen autodissemination and genetic or Wolbachia-based methods, still have to be developed to demonstrate their efficacy and sustainability, but could be considered in future integrated programmes.
It is suggested that mosquito control programmes should be more effective against Ae. aegypti (as opposed to Ae. albopictus) due to its strong urban presence and preference for feeding on humans [13]. Using a combination of control methods as opposed to one strategy is suggested to be most effective, and will reduce the chance of introducing selective pressures – e.g. on oviposition site selection [65]. However, following the discovery of Ae. aegypti in Madeira, using a combined control strategy of spraying insecticides, reducing potential breeding sites and increasing public health awareness did not prevent the species from re-establishing itself there [3].
Existing public health awareness and education materials
ECDC provides regularly-updated vector distribution maps and guidelines for the surveillance of invasive mosquitoes [61].
The US Centers for Disease Prevention and Control (US CDC) provide advice for travellers on protection against mosquitoes, ticks and other arthropods: http://wwwnc.cdc.gov/travel/yellowbook/2010/chapter-2/protection-against-mosquitoes-ticks-insects-arthropods.aspx(link is external).
The National Travel Health Network and Centre provides information on how to avoid insect bites (including mosquito bites): http://www.nathnac.org/pro/factsheets/iba.htm(link is external).
At its 63rd session in September 2013 The Regional Committee for Europe endorsed a ‘Regional framework for surveillance and control of invasive mosquito vectors and re-emerging vector-borne diseases 2014–2020’ [report(link is external)] [resolution(link is external)].
Key areas of uncertainty
It is clear that if Ae. aegypti re-establishes itself in the European regions it previously inhabited and spreads, it will have a significant impact on public health. The spread of Ae. aegypti needs to be monitored as this species is the primary vector of dengue, chikungunya, yellow fever and Zika viruses.
Footnote
*Vector competence is the physiological ability of a mosquito to become infected with and transmit a pathogen, and is typically assessed in laboratory studies. In nature, transmission of a pathogen by vectors is dependent not only on vector competence but also on factors describing the intensity of interaction between the vector, the pathogen and the host in the local environment. Therefore, vector and host densities, geographic distribution, longevity, dispersal and feeding preferences have to be considered to determine the vectorial capacity of a vector population and its role in transmission
Read more
Reverse identification key for mosquito species
References
1. Reiter P. Yellow fever and dengue: a threat to Europe? Eurosurveillance. 2010 Mar 11;15(10):19509.
2. Schaffner F, Mathis A. Dengue and dengue vectors in the WHO European Region: past, present, and scenarios for the future. Lancet Infectious Diseases. 2014;14(12):1271-80.
3. Almeida AP, Goncalves YM, Novo MT, Sousa CA, Melim M, Gracio AJ. Vector monitoring of Aedes aegypti in the Autonomous Region of Madeira, Portugal. Eurosurveillance. 2007 Nov;12(11):E071115 6.
4. Yunicheva YU, Ryabova TE, Markovich NY, Bezzhonova OV, Ganushkina LA, Semenov VB, et al. First data on the presence of breeding populations of the Aedes aegypti L. mosquito in Greater Sochi and various cities of Abkhazia. Meditsinskaia Parazitologiia I Parazitarnye Bolezni 2008;3:40-3.
5. Scholte E, Den Hartog W, Dik M, Schoelitsz B, Brooks M, Schaffner F, et al. Introduction and control of three invasive mosquito species in the Netherlands, July-October 2010. Eurosurveillance. 2010;15(45):19710.
6. Barceló C, Blanda V, del Castillo-Remiro A, Chaskopoulou A, Connelly CR, Ferrero-Gómez L, et al. Surveillance of invasive mosquito species in islands with focus on potential vectors of zoonotic diseases. In: Gutiérrez-López R, Logan JG, Martínez-de la Puente J (editors). Ecology of diseases transmitted by mosquitoes to wildlife. Wageningen: Wageningen Academic Publishers; 2022. (p. 264).
7. Sanidad activa el Sistema de Vigilancia Entomológica ante la detección de ejemplares de Aedes aegypti en Tenerife: Fundacion Canaria para el control de las enfermedades tropicales; [updated 23/12/2022 and 18/01/2023]. Available from: https://funccet.es/mosquito-aedes-aegypti-tenerife/(link is external)
8. Shkurko J. Action plan drawn up for yellow fever mosquito: CyprusMail; 2022. Available from: https://cyprus-mail.com/2022/10/05/action-plan-drawn-up-for-yellow-fever-mosquito/(link is external)
9. Akiner MM, Demirci B, Babuadze G, Robert V, Schaffner F. Spread of the Invasive Mosquitoes Aedes aegypti and Aedes albopictus in the Black Sea Region Increases Risk of Chikungunya, Dengue, and Zika Outbreaks in Europe. PLoS Neglected Tropical Diseases. 2016;10(4):e0004664.
10. European Centre for Disease Prevention and Control (ECDC). The climatic suitability for dengue transmission in continental Europe. Stockholm: ECDC; 2012. Available from: https://www.ecdc.europa.eu/en/publications-data/climatic-suitability-dengue-transmission-continental-europe
11. Wint W, Jones P, Kraemer M, Alexander N, Schaffner F. Past, present and future distribution of the yellow fever mosquito Aedes aegypti: The European paradox. Sci Total Environ. 2022 Nov 15;847:157566.
12. Rogers DJ, Suk JE, Semenza JC. Using global maps to predict the risk of dengue in Europe. Acta Trop. 2014 Jan;129:1-14.
13. Gould EA, Higgs S. Impact of climate change and other factors on emerging arbovirus diseases. Trans R Soc Trop Med Hyg. 2009 Feb;103(2):109-21.
14. Jansen CC, Beebe NW. The dengue vector Aedes aegypti: what comes next? Microbes Infect. 2010 Apr;12(4):272-9.
15. Honorio NA, Codeco CT, Alves FC, Magalhaes MA, Lourenco-De-Oliveira R. Temporal distribution of Aedes aegypti in different districts of Rio de Janeiro, Brazil, measured by two types of traps. J Med Entomol. 2009 Sep;46(5):1001-14.
16. Weaver SC, Reisen WK. Present and future arboviral threats. Antiviral Res. 2010 Feb;85(2):328-45.
17. Paty MC, Six C, Charlet F, Heuze G, Cochet A, Wiegandt A, et al. Large number of imported chikungunya cases in mainland France, 2014: a challenge for surveillance and response. Eurosurveillance. 2014;19(28):20856.
18. European Centre for Disease Prevention and Control (ECDC). Surveillance Atlas of Infectious Diseases Stockholm: European Centre for Disease Prevention; 2016. Available from: http://atlas.ecdc.europa.eu/public/index.aspx?Instance=GeneralAtlas(link is external)
19. Van Bortel W, Dorleans F, Rosine J, Blateau A, Rousset D, Matheus S, et al. Chikungunya outbreak in the Caribbean region, December 2013 to March 2014, and the significance for Europe. 2014;19(13):20759.
20. Sousa CA, Clairouin M, Seixas G, Viveiros B, Novo MT, Silva AC, et al. Ongoing outbreak of dengue type 1 in the Autonomous Region of Madeira, Portugal: preliminary report. Eurosurveillance. 2012;17(49):20333.
21. European Centre for Disease Prevention and Control (ECDC). Epidemiological update: outbreak of dengue in Madeira, Portugal. Stockholm: ECDC; 2013. Available from: http://ecdc.europa.eu/en/press/news/_layouts/forms/News_DispForm.aspx?List=8db7286c-fe2d-476c-9133-18ff4cb1b568&ID=23
22. Holstein M. Dynamics of Aedes aegypti distribution, density and seasonal prevalence in the Mediterranean area. Bull World Health Organ. 1967;36(4):541-3.
23. Soumahoro MK, Fontenille D, Turbelin C, Pelat C, Boyd A, Flahault A, et al. Imported chikungunya virus infection. Emerg Infect Dis. 2010 Jan;16(1):162-3.
24. Eritja R, Escosa R, Lucientes J, Marques E, Roiz D, Ruiz S. Worldwide invasion of vector mosquitoes: present European distribution and challenges in Spain. Biological Invasions 2005;7(1).
25. Brown JE, Scholte EJ, Dik M, Den Hartog W, Beeuwkes J, Powell JR. Aedes aegypti mosquitoes imported into the Netherlands, 2010. Emerg Infect Dis. 2011 Dec;17(12):2335-7.
26. Otero M, Solari HG, Schweigmann N. A stochastic population dynamics model for Aedes aegypti: formulation and application to a city with temperate climate. Bulletin of Mathematical Biology. 2006 Nov;68(8):1945-74.
27. Wilkerson RC, Linton YM, Fonseca DM, Schultz TR, Price DC, Strickman DA. Making Mosquito Taxonomy Useful: A Stable Classification of Tribe Aedini that Balances Utility with Current Knowledge of Evolutionary Relationships. PLoS One. 2015;10(7):e0133602.
28. Reinert JF, Harbach RE, Kitching IJ. Phylogeny and classification of Aedini (Diptera: Culicidae), based on morphological characters of all life stages. Zool J Linn Soc-Lond. 2004 Nov;142(3):289-368.
29. Fontenille D, Diallo M, Mondo M, Ndiaye M, Thonnon J. First evidence of natural vertical transmission of yellow fever virus in Aedes aegypti, its epidemic vector. Trans R Soc Trop Med Hyg. 1997 Sep-Oct;91(5):533-5.
30. Gonçalves Y, Silva J, Biscotto M. On the presence of Aedes (Stegomyia) aegypti Linnaeus, 1762 (Insecta, Diptera, Culicidae) in the island of Madeira (Portugal). Boletim do Museu Municipal do Funchal. 2008;58(322):53-9.
31. Turell MJ, Dohm DJ, Sardelis MR, Oguinn ML, Andreadis TG, Blow JA. An update on the potential of north American mosquitoes (Diptera: Culicidae) to transmit West Nile virus. J Med Entomol. 2005 Jan;42(1):57-62.
32. Saifur RG, Dieng H, Hassan AA, Salmah MR, Satho T, Miake F, et al. Changing domesticity of Aedes aegypti in northern peninsular Malaysia: reproductive consequences and potential epidemiological implications. PLoS One. 2012;7(2):e30919.
33. Scott TW, Takken W. Feeding strategies of anthropophilic mosquitoes result in increased risk of pathogen transmission. Trends in Parasitology. 2012 Mar;28(3):114-21.
34. Barrera R, Amador M, Diaz A, Smith J, Munoz-Jordan JL, Rosario Y. Unusual productivity of Aedes aegypti in septic tanks and its implications for dengue control. Med Vet Entomol. 2008 Mar;22(1):62-9.
35. Medeiros AS, Marcondes CB, De Azevedo PR, Jeronimo SM, e Silva VP, Ximenes Mde F. Seasonal variation of potential flavivirus vectors in an urban biological reserve in north-eastern Brazil. J Med Entomol. 2009 Nov;46(6):1450-7.
36. Juliano SA, Lounibos LP. Ecology of invasive mosquitoes: effects on resident species and on human health. Ecol Lett. 2005 May;8(5):558-74.
37. Ryabova TY, Yunicheva YV, Markovich NY, Ganushkina LA, Orabei VG, Sergiev VP. Detection of Aedes (Stegomyia) aegypti L. mosquitoes in Sochi. Meditsinskaia Parazitologiia i Parazitarnye Bolezni 2005;Jul-Sept:3-5.
38. Larsen JR, Ashley RF. Demonstration of Venezuelan equine encephalomyelitis virus in tissues of Aedes Aegypti. Am J Trop Med Hyg. 1971 Sep;20(5):754-60.
39. de Lamballerie X, Leroy E, Charrel RN, Ttsetsarkin K, Higgs S, Gould EA. Chikungunya virus adapts to tiger mosquito via evolutionary convergence: a sign of things to come? Virol J. 2008;5:33.
40. Aitken TH, Tesh RB, Beaty BJ, Rosen L. Transovarial transmission of yellow fever virus by mosquitoes (Aedes aegypti). Am J Trop Med Hyg. 1979 Jan;28(1):119-21.
41. Thavara U, Tawatsin A, Pengsakul T, Bhakdeenuan P, Chanama S, Anantapreecha S, et al. Outbreak of Chikungunya Fever in Thailand and Virus Detection in Field Population of Vector Mosquitoes, Aedes Aegypti (L.) and Aedes Albopictus Skuse (Diptera: Culicidae). Se Asian J Trop Med. 2009 Sep;40(5):951-62.
42. Mavale M, Parashar D, Sudeep A, Gokhale M, Ghodke Y, Geevarghese G, et al. Venereal transmission of chikungunya virus by Aedes aegypti mosquitoes (Diptera: Culicidae). Am J Trop Med Hyg. 2010 Dec;83(6):1242-4.
43. Vega-Rua A, Lourenco-de-Oliveira R, Mousson L, Vazeille M, Fuchs S, Yebakima A, et al. Chikungunya virus transmission potential by local Aedes mosquitoes in the Americas and Europe. PLoS Neglected Tropical Diseases. 2015 May;9(5):e0003780.
44. Staples JE, Breiman RF, Powers AM. Chikungunya fever: an epidemiological review of a re-emerging infectious disease. Clin Infect Dis. 2009 Sep 15;49(6):942-8.
45. Zayed A, Awash AA, Esmail MA, Al-Mohamadi HA, Al-Salwai M, Al-Jasari A, et al. Detection of Chikungunya virus in Aedes aegypti during 2011 outbreak in Al Hodayda, Yemen. Acta Trop. 2012 Jul;123(1):62-6.
46. Dupont-Rouzeyrol M, Caro V, Guillaumot L, Vazeille M, D’Ortenzio E, Thiberge JM, et al. Chikungunya virus and the mosquito vector Aedes aegypti in New Caledonia (South Pacific Region). Vector Borne and Zoonotic Diseases. 2012;12(12):1036-41.
47. Roth A, Mercier A, Lepers C, Hoy D, Duituturaga S, Benyon E, et al. Concurrent outbreaks of dengue, chikungunya and Zika virus infections – an unprecedented epidemic wave of mosquito-borne viruses in the Pacific 2012-2014. Eurosurveillance. 2014;19(41):20929.
48. Ramchurn SK, Moheeput K, Goorah SS. An analysis of a short-lived outbreak of dengue fever in Mauritius. Eurosurveillance. 2009;14(34):19314.
49. Gratz NG. Critical review of the vector status of Aedes albopictus. Med Vet Entomol. 2004 Sep;18(3):215-27.
50. Mulyatno KC, Yamanaka A, Yotopranoto S, Konishi E. Vertical transmission of dengue virus in Aedes aegypti collected in Surabaya, Indonesia, during 2008-2011. Japanese Journal of Infectious Diseases. 2012;65(3):274-6.
51. Stanaway JD, Shepard DS, Undurraga EA, Halasa YA, Coffeng LE, Brady OJ, et al. The global burden of dengue: an analysis from the Global Burden of Disease Study 2013. Lancet Infectious Diseases. 2016 Jun;16(6):712-23.
52. Rosen L. Dengue in Greece in 1927 and 1928 and the pathogenesis of dengue hemorrhagic fever: new data and a different conclusion. The American Journal of Tropical Medicine and Hygiene. 1986 May;35(3):642-53.
53. Monath TP, Cetron MS. Prevention of yellow fever in persons traveling to the tropics. Clin Infect Dis. 2002 May 15;34(10):1369-78.
54. World Health Organization (WHO). List of countries, territories and areas. Yellow fever vaccination requirements and recommendations; malaria situation; and other vaccination requirements. Geneva: WHO; 2015.
55. Agampodi SB, Wickramage K. Is there a risk of yellow fever virus transmission in South Asian countries with hyperendemic dengue? Biomed Res Int. 2013;2013:905043.
56. Musso D, Gubler DJ. Zika Virus. Clin Microbiol Rev. 2016 Jul;29(3):487-524.
57. Ferreira-de-Brito A, Ribeiro IP, Miranda RM, Fernandes RS, Campos SS, Silva KA, et al. First detection of natural infection of Aedes aegypti with Zika virus in Brazil and throughout South America. Memórias do Instituto Oswaldo Cruz. 2016 Oct;111(10):655-8.
58. Chouin-Carneiro T, Vega-Rua A, Vazeille M, Yebakima A, Girod R, Goindin D, et al. Differential Susceptibilities of Aedes aegypti and Aedes albopictus from the Americas to Zika Virus. Plos Neglected Tropical Diseases. 2016 Mar;10(3):e0004543.
59. Li MI, Wong PS, Ng LC, Tan CH. Oral susceptibility of Singapore Aedes (Stegomyia) aegypti (Linnaeus) to Zika virus. Plos Neglected Tropical Diseases. 2012;6(8):e1792.
60. Diagne CT, Diallo D, Faye O, Ba Y, Faye O, Gaye A, et al. Potential of selected Senegalese Aedes spp. mosquitoes (Diptera: Culicidae) to transmit Zika virus. BMC Infectious Diseases. 2015;15:492.
61. European Centre for Disease Prevention and Control (ECDC). Guidelines for the surveillance of invasive mosquitoes in Europe. Stockholm: ECDC; 2012. Available from: https://www.ecdc.europa.eu/en/publications-data/guidelines-surveillance-invasive-mosquitoes-europe
62. Arredondo-Jimenez JI, Valdez-Delgado KM. Aedes aegypti pupal/demographic surveys in southern Mexico: consistency and practicality. Ann Trop Med Parasitol. 2006 Apr;100 Suppl 1:S17-S32.
63. Gubler DJ. Resurgent vector-borne diseases as a global health problem. Emerg Infect Dis. 1998 Jul-Sep;4(3):442-50.
64. Baldacchino F, Caputo B, Chandre F, Drago A, della Torre A, Montarsi F, et al. Control methods against invasive Aedes mosquitoes in Europe: a review. Pest Management Science. 2015 Nov;71(11):1471-85.
65. Wong J, Morrison AC, Stoddard ST, Astete H, Chu YY, Baseer I, et al. Linking oviposition site choice to offspring fitness in Aedes aegypti: consequences for targeted larval control of dengue vectors. PLoS Neglected Tropical Diseases. 2012;6(5):e1632.
Source: European Centre Disease Control (ECDC)
Aedes albopictus (Tiger Mosquito) – Factsheet for experts

- SPECIES NAME/CLASSIFICATION: Aedes (Stegomyia) albopictus (Skuse) [66]
- COMMON NAME: Asian tiger mosquito, Forest day mosquito
- SYNONYMS AND OTHER NAME IN USE: Stegomyia albopicta (sensu Reinert et al. [67])
This mosquito species is a known vector of chikungunya virus, dengue virus and dirofilariasis.
Hazard associated with mosquito species
Current issues
Invasive species/global dispersion
Aedes albopictus has undergone a dramatic global expansion facilitated by human activities, in particular the movement of used tyres and ‘lucky bamboo’ [1]. Together with passive transit via public and private transport, this has resulted in a widespread global distribution of Ae. albopictus. It is now listed as one of the top 100 invasive species by the Invasive Species Specialist Group [2].
Ecological plasticity
The success of the invasion of Ae. albopictus is due to a number of factors including: its ecological plasticity, strong competitive aptitude, globalization i.e. increase of trade and travel, lack of surveillance, and lack of efficient control [1]. Climate change predictions suggest Ae. albopictus will continue to be a successful invasive species that will spread beyond its current geographical boundaries [3-5]. This mosquito is already showing signs of adaptation to colder climates [1,6] which may result in disease transmission in new areas.
Biting and disease risk
Aedes albopictus feeds on a wide range of hosts. It is also known to be a significant biting nuisance, with the potential to become a serious health threat as a bridge vector of zoonotic pathogens to humans [7]. This mosquito species is a known vector of chikungunya virus, dengue virus and dirofilariasis. A number of other viruses affecting human health have also been isolated from field-collected Ae. albopictus in different countries. Moreover, its recent involvement in the localised transmission of chikungunya virus in Italy [8] and France [9,10] and dengue virus in France [11-13] and Croatia [14] highlights the importance of monitoring this invasive species.
Geographical distribution
Aedes albopictus has been reported in the following areas: [1,3,6,7,15-42].
- Europe: Albania, Austria (not established to date), Belgium (not established to date), Bosnia & Herzegovina, Bulgaria, Croatia, Czech Republic (not established to date), France (including Corsica), Georgia, Germany, Greece, Hungary, Italy (including Sardinia, Sicily, Lampedusa, and other islands), Malta, Monaco, Montenegro, the Netherlands (not established to date), Romania, Russia, San Marino, Serbia (not established to date), Slovakia (not established to date), Slovenia, Spain, Switzerland, Turkey and Vatican City
- Middle East: Israel, Lebanon, Saudi Arabia (to be confirmed), Syria, Yemen (to be confirmed)
- Asia & Australasia: Australia (established only in the Torres Strait, the region that separates mainland Australia from Papua New Guinea), Japan, New Zealand (not established), numerous Pacific Ocean and Indian Ocean islands, southern Asia
- North, Central America & Caribbean: Barbados (not established), Belize, Cayman Islands, Costa Rica, Cuba, Dominican Republic, El Salvador, Guatemala, Haiti, Honduras, Mexico, Nicaragua, Panama, Trinidad (not established), USA
- South America: Argentina, Bolivia (not confirmed), Brazil, Colombia, Paraguay, Uruguay, Venezuela
- Africa: Algeria, Cameroon, Central African Republic, Equatorial Guinea, Gabon, Madagascar, Nigeria, Republic of Congo, South Africa (not established)
The current known distribution of Ae. albopictus in Europe in displayed on the vector maps
Brief history of spread and European distribution
Pathways
Having originated in tropical forests of South-East Asia, Ae. albopictus has spread globally. This geographical spread has mostly occurred during the past three decades [1] via passive transport of eggs in used tyres or lucky bamboo, the latter being the route of importation into Belgium, the Netherlands and California [16,43,44]. Public or private transport from heavily-infested areas has also resulted in the passive transportation of Ae. albopictus into new areas. Passive transport from heavily infested areas via ground vehicles is believed to be the route of introduction of Ae. albopictus into southern France, Germany, the Balkans, the Czech Republic, Spain and Switzerland [19,23,30,45].
Timeline of initial movements
Aedes albopictus was first reported in Europe in 1979 in Albania [46]. In 1985 it was reported in Texas, USA and has since spread northward and eastward, having now been reported in at least 32 US states including Hawaii [38]. This expansion was facilitated by the movement of used tyres along the interstate highways [18]. In Latin America it was first reported in Brazil in 1986 and later in Mexico in 1988 [19]. In Africa, it was first detected in 1990 in South Africa but establishment was only reported in 2000 from Cameroon [47,48].
Initial importation and spread in Europe
The first record of importation to Europe was in Albania in 1979 but it was suspected to be present from 1976. Although Ae. albopictus became established in Albania, there were no reports in any other European country until 1990, when it was found in Italy [49]. Since its importation into Italy through Genoa [23], Ae. albopictus has now become established in most areas of the country <600m above sea level and is abundant in many urban areas [3,50]. During the first 10 years of colonisation in the country, Ae. albopictus spread throughout 22 provinces, mainly in the north east of the country [51]. Italy is now the most heavily-infested country in Europe, with the highest incidence in the Veneto and Friuli-Venezia-Giulia regions, large parts of Lombardia and Emilia-Romagna and coastal areas of central Italy [3]. The mosquito was then reported in France in 1999 and Belgium in 2000. These initial importations were subsequently eradicated or died out, but spread has occurred to a number of countries in Europe since 2000, including southern France.
Having become established in Albania, Italy and on the Cote d’Azur in France, Ae. albopictus is also known to be spreading in Greece, Spain and the Balkan countries [23]. Aedes albopictus has also been reported in Ticino in Switzerland since 2003, suggesting sporadic introductions from Italy [52]. In 2004, it was reported near Barcelona in Spain, with some spread along the Mediterranean coast [19]. Furthermore, it has been repeatedly found in the Netherlands (2005, 2006 and 2007) at the premises of companies importing bamboo [43,53] and in Malta [26]. The Dutch populations, imported with lucky bamboo, have not established outside greenhouses, suggesting that they are tropical strains. Besides, populations are also imported from USA via used tyre trade, and control measures have so far avoided their establishment [54]. Aedes albopictus has been trapped on a number of occasions along motorways in southern Germany, suggesting introduction by vehicles from southern Europe [32,45,55]. In 2014, all developmental stages were found over extended periods of time in southern Germany indicating local reproduction [56].
Further specimens were collected at parking lots along motorways and at some other places in Austria [31], Czech Republic [30], and Slovakia [33], but subsequent surveys remained negative [38]. Finally, populations have been found established in Slovenia in 2007 [57,58], in Bulgaria in 2011 [59], Russia in 2011 [60,61], Turkey in 2011 [35] and Romania in 2012 [40].
Possible future expansion
The ability for imported populations to establish is currently dependent on the origin of the mosquito and its strain and it is not always clear whether introductions into Europe will result in established populations. In this context, it is suggested that Portugal, the eastern Adriatic Coast, eastern Turkey and the Caspian Sea coast of Russia are the most likely places for Ae. albopictus to establish itself in Europe [7]. Risk mapping projections suggest that further expansion of this species will occur in the Mediterranean basin towards the east and the west, as well as in the coastal areas of Greece, Turkey and the Balkan countries. Incorporation of climate change projections suggests that over time most of Europe will become more suitable for Ae. albopictus establishment [3,62,63]. Especially Western Europe (Belgium, France, Luxembourg and the Netherlands) will provide favourable climatic conditions within the next decades. Climatic conditions will continue to be suitable in southern France as well as in most parts of Italy and Mediterranean coastal regions in south-eastern Europe [63]. It is predicted that future climate trends will increase the risk of establishment in northern Europe, e.g. parts of Germany and the southernmost parts of the UK, due to wetter and warmer conditions, and slightly decrease the risk across southern Europe because of hotter and drier summers [3,62,63]. Land use changes, particularly urbanisation, may continue to increase the competitive advantage of Ae. albopictus over resident mosquitoes through its exploitation of artificial container habitats; further aiding establishment in new areas [64]. Winter temperatures and mean annual temperatures appear to be the most significant limiting factors of Ae. albopictus expansion in Europe [65].
Entomology
- SPECIES NAME/CLASSIFICATION: Aedes (Stegomyia) albopictus (Skuse) [66]
- COMMON NAME: Asian tiger mosquito, Forest day mosquito
- SYNONYMS AND OTHER NAME IN USE: Stegomyia albopicta (sensu Reinert et al. [67])
Morphological characters and similar species
Aedes albopictus adults are relatively small and show a black and white pattern due to the presence of white/silver scale patches against a black background on the legs and other parts of the body. Some indigenous mosquitoes also show such contrasts but these are less obvious (more brownish and yellowish). Aedes albopictus can, however, be confused with other invasive (Ae. aegypti, Ae. japonicus) or indigenous species (Ae. cretinus, restricted to Cyprus, Greece and Turkey), and the diagnostic character is the presence of a median silver-scale line against a black background on the scutum (dorsal part of the thorax). The differentiation with Ae. cretinus needs a detailed check of scale patches on the thorax.
Life history
Diapausing tendencies
Tropical and subtropical populations are active throughout the year with no diapausing phase [26]. Temperate populations are affected by seasonal temperature and photoperiodicity and, in response to these factors, can overwinter by producing eggs that undergo a winter diapause [68]. Eggs, laid during late summer or early autumn when daylight hours are reducing, enter facultative diapause, and hatching suppression occurs which is usually sufficient to outlast winter [68]. The species’ ability to induce photoperiodic egg diapause allows it to overwinter in temperate regions, which assists its establishment in more northern latitudes in Asia, North America and Europe. Diapausing eggs of European Ae. albopictus have been shown to be able to survive a cold spell of -10oC, whereas eggs of tropical Ae. albopictus could only survive -2oC. In addition, the hatching success and cold tolerance of European Ae. albopictus eggs were increased in diapausing eggs when compared to non-diapausing eggs [69]. Aedes albopictus populations in Italy are showing signs of cold-acclimation as adults and are thus remaining active throughout winter [70]. Some populations in North America are likely to be exposed to mean temperatures of -5˚C and will overwinter if females have deposited eggs in containers that are not exposed to these temperatures for prolonged periods ‒ e.g. artificial containers in peridomestic areas [64].
General life history
The drought-resistant eggs are laid above the water line. Larval/pupal development takes three to eight weeks and is continuous throughout the year in European southernmost regions (Malta [26]). Adult females can survive over three weeks [70]. They have been reported to overwinter in Rome [70] and even to lay eggs during winter time in Spain [71].
Seasonal abundance
Seasonal abundance is dependent on temperature and the availability of food and water in a particular geographical area. Higher temperatures speed up larval development, increasing the number of adult populations, the autumnal development of immatures and consequently the rates of egg overwintering [68]. A study in northern Italy showed an increased abundance of adult females during the period May-September, peaking in late July [72]. In Greece, Ae. albopictus is continuously active for over eight months of the year with the greatest abundance during summer and autumn, peaking in October. Oviposition takes place from mid-April to December, with the numbers of eggs highest from mid-July to the end of the autumn, and significantly increased during mild and rainy weather [73].
Voltinism (generations per season)
Multivoltine, 5-17 generations per year [26].
Host preferences
Aedes albopictus is an opportunistic feeder [74]. Blood hosts include humans, domestic and wild animals, reptiles, birds and amphibians [18]. Yet, laboratory studies and blood meal analysis have shown a preference for human blood meals [1]. A study in Italy found a preference for mammals as opposed to birds and found human blood meals were more frequent in urban areas than rural sites, suggesting that host availability and abundance has a direct impact on the feeding activities of Ae. albopictus [50].
Aquatic and terrestrial habitats
Aedes albopictus has the ability to breed in natural and artificial habitats, some of which include tyres, barrels, rainwater gulley catch basins and drinking troughs [26]. Natural habitats consist in phytotelms (water bodies held by terrestrial plants e.g. tree holes) and rock pools [75]. They are not known to breed in brackish or salt water [24]. In general, albeit in Europe, they have a preference for urban and suburban habitats [76]. Aedes albopictus is said to be superior in competing for food resources with Ae. triseriatus and Ae. japonicus [64].
Biting and resting habits
Aedes albopictus is currently considered a serious biting nuisance for humans in Italy [23,77], southern France [78] and Spain where it is significantly reducing the quality of life in infested areas [19]. Adult females bite aggressively, usually during the day and preferably outdoors. However, there are reports that Ae. albopictus is becoming partially endophilic [77], and is found to be biting indoors [79]. During a study in Rome, blood-fed females were mainly found indoors, indicating that local mosquito populations could spend time resting indoors after a blood meal [50]. Another study on Penang Island in Malaysia reported observations of Ae. albopictus females developing indoors within containers. Such containers included flower vases, empty paint cans and sinks. Most stages of larval populations were present over a five-month period, suggesting that this species may have adapted to indoor environments [80]. A laboratory study found that Ae. albopictus could survive for long periods indoors by obtaining sugars from lucky bamboo and other ornamental plants [81]. The mosquitoes’ survival time was long enough to complete a gonotrophic cycle, and to allow development of transmissible arboviruses within the vector [81].
Environmental thresholds/constraints/development criteria
Establishment thresholds
The extent to which Ae. albopictus has established itself in new geographic locations is thought to correspond to various climatic thresholds: a mean winter temperature of >0oC to permit egg overwintering, a mean annual temperature of >11oC required for adult survival and activity, and at least 500mm of annual rainfall; a pre-requisite for the maintenance of aquatic habitats. However, rainfall needs to be sufficient during summer months to maintain such sites [68,82]. Conversely, periods of high precipitation reduce short-term abundance of host-seeking females [72]. A summer temperature of 25‒30oC is required for optimum development [83]. However, there are reports of populations establishing in areas with lower mean temperatures (5‒28.5°C) and lower rainfall (290mm annually) than previously suspected [7,84].
Diapausing and reactivation cues
The length of the reproductive season is regulated by the increasing temperatures in spring and the onset of egg diapause in autumn. The critical photoperiodic threshold varies between geographical locations. In general, the production of diapausing eggs occurs below 13‒14 hours of daylight, however in some locations this threshold occurs at 11‒12 hours [68].
Hatching of diapausing eggs in spring is related to changes in photoperiod (i.e. length of day), food availability, temperature, and water availability. Furthermore, this mosquito may not survive through winter if environmental temperatures and humidity are not maintained above set thresholds, or if the diapause period exceeds six months [68].
There is generally little adult activity below 9oC, but adults do seek warmer microclimates indoors [72]. In parts of Italy, adult activity continues throughout winter [70].
Dispersal range
Flight range (and hence dispersal on the wing) is limited to ~200m [74]. The main dispersal route for this mosquito is via transport and movement of container habitat goods.
Epidemiology and transmission of pathogens
Known vector status
During the 2006-2007 chikungunya outbreak in Italy, the status of Ae. albopictus as vector of the chikungunya virus was clearly demonstrated [1]. This mosquito is also known to be able to transmit dengue virus [85,86] and dirofilarial worms [19,87]. All four dengue virus serotypes have been isolated from Ae. albopictus [88]. Infection studies of l Ae. albopictus suggest a possible contribution to Zika virus outbreaks [89,90].
Aedes albopictus is considered to be a competent* vector experimentally of at least 22 other arboviruses including yellow fever virus, Rift Valley fever virus, Japanese encephalitis virus, West Nile virus and Sindbis virus, all of which are relevant to Europe. Potosi virus, Cache Valley virus, La Crosse virus, Eastern equine encephalitis virus, Mayaro virus, Ross River virus, Western equine encephalitis virus, Venezuelan equine encephalitis virus, Oropouche virus, Jamestown Canyon virus, San Angelo virus and Trivittatus virus are other arboviruses that Ae. albopictus can transmit experimentally [38,91].
A number of these viruses have also been isolated from field-collected Ae. albopictus in different countries and laboratory transmission of such viruses by Ae. albopictus has been demonstrated [1]. These include Eastern equine encephalitis virus [92,93], La Crosse virus [94,95], Venezuelan equine encephalitis virus [96,97], West Nile virus [72,98,99] and Japanese encephalitis virus [1]. Usutu virus has been isolated from Ae. albopictus in Italy, but it is unknown whether the mosquito can transmit this pathogen [100]. Field isolation and experimental infection studies alone do not prove that this mosquito species is involved in the transmission of such viruses, but the mosquito’s biting habits, increasing global distribution and recent involvement in a chikungunya virus outbreak highlight the significance of Ae. albopictus to public health.
A high prevalence of the insect-infective Aedes flavivirus has been detected in Ae. albopictus in Italy and it has been suggested that its presence in these mosquitoes could influence the transmission dynamics of other human-pathogenic flaviviruses, such as West Nile virus and Usutu virus [101].
Not only does Ae. albopictus represent a disease risk but it can also cause a considerable amount of nuisance biting in areas where it is well-established, reducing the quality of life of individuals affected [102]. Prevalence of Ae. albopictus has also been linked to a reduction in children’s outdoor physical activity time, a factor contributing to childhood obesity [103].
Chikungunya
Aedes albopictus mosquitoes are able to transmit chikungunya virus within two days of ingesting a viraemic blood meal [104]. Some experts suggest that transovarial transmission is enough to maintain viral cycles but others disagree [23]. No evidence of transovarial transmission was found during an entomological investigation into the 2007 chikungunya outbreak in Italy [105] but the virus was detected in field-caught male Ae. albopictus following an outbreak in Thailand [106].
Chikungunya was first reported in Europe in 2007 following epidemics in the Indian Ocean (2005‒2007), which caused millions of cases and significant morbidity and burden on health resources. This was the first known local transmission of chikungunya in Europe and occurred in Emilia-Romagna province in Italy. An infected traveller returned home from India, spreading the disease to localised populations of Ae. albopictus mosquitoes. Following entomological investigations during the outbreak females of Ae. albopictus were found to be PCR positive and the virus was successfully isolated [105]. The adaptation of the virus to this new vector host (in addition to its principle vector Ae. aegypti) has resulted in improved virus replication and transmission efficiency of the virus by Ae. albopictus [5,107] [104]. Autochthonous chikungunya fever cases occurred in south eastern France in 2010 and 2014 [9,10]. Tilston et al considers that, based on temperature, the southern European countries are most at risk of chikungunya virus transmission [108].
Dengue
Although generally considered a secondary vector of dengue to Ae. aegypti, Ae. albopictus has been associated with dengue virus transmission and this has been acknowledged since the mid-nineteenth century [1]. It was implicated as the vector responsible for outbreaks in Hawaii [85] Reunion Island and Mauritius [86,109] . It has also been associated with dengue virus transmission in China, Japan and Seychelles [88]. Dengue virus is transmitted transovarially so emergence of adults from imported infected eggs could lead to further spread of the disease [24]. Dengue virus can also be transmitted venereally in mosquitoes [88].
Autochthonous cases of dengue were reported in France during September 2010 [11] followed by others in Croatia at around the same time [14]. Further cases, linked to Ae. albopictus, were reported from France in 2013, 2014 and 2015 [12,13,110]. Although modelling predicts that most of Europe is currently unsuitable for dengue transmission, areas combining high human population density with suitable day- and night-time land surface temperatures are still at greater risk [4,111]. However, competent mosquito vectors must be present for transmission to occur. Climatically, areas predicted to be at risk from dengue include northern Italy, parts of Austria, Slovenia and Croatia, and west of the Alps in France [111]. The risk of transmission to humans is considered to be higher where there is a presence of Ae. aegypti than in areas with Ae. albopictus. This point is exemplified by the outbreak of dengue on Madeira associated with Ae. aegypti [112].
Zika
Aedes albopictus is considered a potential vector of Zika virus. Vector competence studies of local Ae. albopictus in Singapore with the African lineage of Zika virus showed the potential of this mosquito to transmit Zika virus [89]. Recent studies using different Ae. albopictus populations from the Americas and Europe revealed that this mosquito is susceptible to Zika virus infection, that the virus is disseminated and can reach the salivary glands but not very efficiently; Ae. albopictus has a lower vector competence compared to Aedes aegypti [113] [114] [115]. The species has been found infected in wild caught mosquitoes [90].
Dirofilariasis
Aedes albopictus has a role in the transmission of Dirofilaria in Asia, North America and Europe [1]. Dirofilaria (filarial nematodes D. immitis and D. repens) is a parasite transmitted primarily between dogs (or other canids which act as reservoir hosts) and mosquitoes, but which can also affect humans. Recent evidence has shown transmission of the parasite by Italian Ae. albopictus populations [116-118], coupled with an increase in prevalence of human dirofilariasis in Italy [87].
Human infections are increasing in Europe and although it is unusual for the parasite to develop into the adult stage in humans, at least three cases of microfilaraemic zoonotic infections have been reported in Europe [77,119].
Factors driving/impacting on transmission cycles
A growth in dengue cases worldwide, increasing global travel and established populations of Ae. albopictus may have been the cause of the dengue outbreak in Mauritius in June 2009 [86]. The movement of viraemic hosts can result in outbreaks of chikungunya virus in non-endemic areas. Climate change could increase the distribution of Ae. albopictus beyond its current boundaries which could enhance the transmission potential of chikungunya virus and dengue virus in temperate regions [5,77,120]. Aedes albopictus mosquitoes tend to feed on multiple hosts which also increases the risk of zoonotic disease transmission [1]. The return of viraemic travellers from disease-epidemic areas to temperate regions has resulted in (and will potentially continue to result in) local mosquito populations sustaining disease transmission [83]. Therefore, the presence of Ae. albopictus in Europe and the increasing number of overseas travellers may increase the risk of dengue and chikungunya outbreaks in Europe [121].
Public health (control/interventions)
Vector surveillance
Methods for surveying Ae. albopictus are addressed in the ‘ECDC Guidelines for the surveillance of invasive mosquitoes in Europe [122].
ECDC and EFSA fund European-wide monitoring and mapping activities for invasive mosquito species and potential mosquito vectors (VectorNet).
Species specific control methods
Source reduction and adult control
Control of Ae. albopictus is based on the reduction of larval development sites. Mosquito fogging and larviciding (insecticides targeting the mosquito larvae) were techniques used during an outbreak of dengue in Mauritius in June 2009 [86]. In 2006, use of insecticides in greenhouses that had been recently colonised by Ae. albopictus in the Netherlands may have contributed to the decline in numbers caught the following year [43]. Permethrin, Bacillus thuringiensis israeliensis ser. H14 and diflubenzuron (an insect growth regulator) were used to treat stagnant water after the detection of Ae. albopictus in Switzerland in 2003 [52]. Although resistance to insecticides is not currently a problem, it has been detected in a population in Thailand [123] and more recently in populations in La Reunion [124] and Malaysia [123,125]. In Pakistan, field-collected Ae. albopictus displayed moderate-high resistance to many agricultural insecticides, including pyrethroids [126].
Control of this species in newly-established areas has been difficult (e.g. USA, France and Italy) [1]. Although prevention of invasion was achieved after its first introduction into France in 1999, subsequent introductions (most likely by vehicles from Italy) have resulted in Ae. albopictus becoming a pest problem in southern France and Corsica. A study in Catalonia, Spain demonstrated the use of multiple intervention strategies (source reduction, larvicide and adulticide treatments and the cleaning of uncontrolled landfills) as successful in curbing an established population of Ae. albopictus (produced a marked reduction in egg numbers). The authors concluded that citizen cooperation was an essential component for successfully implementing these interventions [102].
Implementation of an integrated control strategy against invasive mosquito species should take into account the target species, its ecology and the public health concern, i.e. nuisance or disease transmission. As a general rule, an integrated Invasive Mosquito Species control strategy requires the coordinated involvement of local authorities, private partners, organised society and communities [127].
Decreasing human-vector contact and the use of public health material have been widely used in endemic areas in Europe, such as Italy. The use of irradiated or genetically modified mosquitoes which are still under development are methods that may be used in the future to complement conventional methods. Additional control methods which may be applied in the future include Wolbachia infection to block transmission of dengue virus and chikungunya virus, and the introduction of natural predators [34]. Personal protective measures to reduce the risk of mosquito bites include the use of mosquito bed nets (preferably insecticide-treated nets), sleeping or resting in screened or air-conditioned rooms, the wearing of clothes that cover most of the body, and the use of mosquito repellent in accordance with the instructions indicated on the product label.
Existing public health awareness and education materials
- ECDC also provides information on dengue, chikungunya, yellow fever and Zika virus.
- The ECDC provides updated vector distribution maps and step-by-step web guidelines for the surveillance of invasive mosquitoes.
- The CDC provides advice for travellers on protection against mosquitoes, ticks and other arthropods.
- The National travel health network and centre provides information on how to avoid insect bites (including mosquito bites).
- The Regional Committee for Europe has endorsed at his 63rd session, September 2013, a ‘Regional framework for surveillance and control of invasive mosquito vectors and re-emerging vector-borne diseases 2014–2020’ [report(link is external)] [resolution(link is external)
Key areas of uncertainty
Although it is not clear how significant Ae. albopictus will be in disease transmission across Europe, the ability of Ae. albopictus to adapt to new environments, its predicted spread and establishment in Europe and its confirmed involvement in disease transmission cycles makes the surveillance and control of this species hugely important.
Footnote
*Vector competence is the physiological ability of a mosquito to become infected with and transmit a pathogen, and is typically assessed in laboratory studies. In nature, transmission of a pathogen by vectors is dependent not only on vector competence but also on factors describing the intensity of interaction between the vector, the pathogen and the host in the local environment. Therefore, vector and host densities, geographic distribution, longevity, dispersal and feeding preferences have to be considered to determine the vectorial capacity of a vector population and its role in transmission
Read more
Reverse identification key for mosquito species
References
1. Paupy C, Delatte H, Bagny L, Corbel V, Fontenille D. Aedes albopictus, an arbovirus vector: from the darkness to the light. Microbes Infect. 2009 Dec;11(14-15):1177-85.
2. Invasive Species Specialist Group. Global Invasive Species Database – Aedes albopictus 2009. Available from: http://www.issg.org/database/species/ecology.asp?si=109&fr=1&sts=sss&lang=EN(link is external).
3. European Centre for Disease Prevention. Development of Aedes albopictus risk maps. Stockholm: European Centre for Disease Prevention and Control, 2009.
4. European Centre for Disease P, Control. The climatic suitability for dengue transmission in continental Europe. Stockholm: ECDC; 2012.
5. Gould EA, Higgs S. Impact of climate change and other factors on emerging arbovirus diseases. Trans R Soc Trop Med Hyg. 2009 Feb;103(2):109-21.
6. Kraemer MU, Sinka ME, Duda KA, Mylne AQ, Shearer FM, Barker CM, et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. Elife. 2015;4:e08347.
7. Benedict MQ, Levine RS, Hawley WA, Lounibos LP. Spread of the tiger: global risk of invasion by the mosquito Aedes albopictus. Vector Borne Zoonotic Dis. 2007 Spring;7(1):76-85.
8. Rezza G, Nicoletti L, Angelini R, Romi R, Finarelli AC, Panning M, et al. Infection with chikungunya virus in Italy: an outbreak in a temperate region. Lancet. 2007 Dec 1;370(9602):1840-6.
9. Grandadam M, Caro V, Plumet S, Thiberge JM, Souares Y, Failloux AB, et al. Chikungunya virus, southeastern France. Emerg Infect Dis. 2011 May;17(5):910-3.
10. Delisle E, Rousseau C, Broche B, Leparc-Goffart I, L’Ambert G, Cochet A, et al. Chikungunya outbreak in Montpellier, France, September to October 2014. Euro Surveill. 2015;20(17):21108.
11. La Ruche G, Souares Y, Armengaud A, Peloux-Petiot F, Delaunay P, Despres P, et al. First two autochthonous dengue virus infections in metropolitan France, September 2010. Euro Surveill. 2010 Sep 30;15(39):19676.
12. Marchand E, Prat C, Jeannin C, Lafont E, Bergmann T, Flusin O, et al. Autochthonous case of dengue in France, October 2013. Euro Surveill. 2013;18(50):20661.
13. Succo T, Leparc-Goffart I, Ferre JB, Roiz D, Broche B, Maquart M, et al. Autochthonous dengue outbreak in Nimes, South of France, July to September 2015. Euro Surveill. 2016 May 26;21(21):30240.
14. Gjenero-Margan I, Aleraj B, Krajcar D, Lesnikar V, Klobucar A, Pem-Novosel I, et al. Autochthonous dengue fever in Croatia, August-September 2010. Euro Surveill. 2011;16(9).
15. Schaffner F, Karch S. [First report of Aedes albopictus (Skuse, 1984) in metropolitan France]. C R Acad Sci III. 2000 Apr;323(4):373-5.
16. Madon MB, Mulla MS, Shaw MW, Kluh S, Hazelrigg JE. Introduction of Aedes albopictus (Skuse) in southern California and potential for its establishment. J Vector Ecol. 2002 Jun;27(1):149-54.
17. Schaffner F, Van Bortel W, Coosemans M. First record of Aedes (Stegomyia) albopictus in Belgium. J Am Mosq Control Assoc. 2004 Jun;20(2):201-3.
18. Eritja R, Escosa R, Lucientes J, Marques E, Roiz D, Ruiz S. Worldwide invasion of vector mosquitoes: present European distribution and challenges in Spain. Biol Invasions. 2005;7(1).
19. Aranda C, Eritja R, Roiz D. First record and establishment of the mosquito Aedes albopictus in Spain. Med Vet Entomol. 2006 Mar;20(1):150-2.
20. Contini C. Aedes albopictus in Sardinia: reappearance or widespread colonization? Parassitologia. 2007 Jun;49(1-2):33-5.
21. Haddad N, Harbach RE, Chamat S, Bouharoun-Tayoun H. Presence of Aedes albopictus in Lebanon and Syria. J Am Mosq Control Assoc. 2007 Jun;23(2):226-8.
22. Haddad N, Mousson L, Vazeille M, Chamat S, Tayeh J, Osta MA, et al. Aedes albopictus in Lebanon, a potential risk of arboviruses outbreak. BMC Infectious Diseases 2012;12:300.
23. Scholte EJ, Schaffner F. Waiting for the tiger: establishment and spread of the Aedes albopictus mosquito in Europe. In: Takken W, Knols BGJ, editors. Emerging pests and vector-borne diseases in Europe. 1. Wageningen, The Netherlands: Wageningen Academic Publishers, ; 2007. p. 241-60.
24. Buhagiar JA. A second record of Aedes (Stegomyia) albopictus (Diptera: Culicidae) in Malta. Eu Mosq Bull. 2009;27:65-7.
25. Diallo M, Laganier R, Nangouma A. First record of Ae. albopictus (Skuse 1894), in Central African Republic. Trop Med Int Health. 2010;15(10):1185-9.
26. Gatt P, Deeming JC, Schaffner F. First records of Aedes (Stegomyia) albopictus (Skuse) (Diptera: Culicidae) in Malta. Eu Mosq Bull. 2009;27 56-64.
27. Carrieri M, Albieri A, Angelini P, Baldacchini F, Venturelli C, Zeo SM, et al. Surveillance of the chikungunya vector Aedes albopictus (Skuse) in Emilia-Romagna (northern Italy): organizational and technical aspects of a large scale monitoring system. J Vector Ecol. 2011 Jun;36(1):108-16.
28. Izri A, Bitam I, Charrel RN. First entomological documentation of Aedes (Stegomyia) albopictus (Skuse, 1894) in Algeria. Clin Microbiol Infect. 2011;17(7):1116-8.
29. Fernandez Mdel C, Jean YS, Callaba CA, Lopez LS. The first report of Aedes (Stegomyia) albopictus in Haiti. Memórias do Instituto Oswaldo Cruz. 2012 Mar;107(2):279-81.
30. Sebesta O, Rudolf I, Betasova L, Pesko J, Hubalek Z. An invasive mosquito species Aedes albopictus found in the Czech Republic, 2012. Euro Surveill. 2012;17(43):20301.
31. Seidel B, Duh D, Nowotny M, Allerberger F. Erstnachweis der Stechmücken Aedes (Ochlerotatus) japonicus japonicus (Theobald, 1901) in Österreich und Slowenien in 2011 und für Aedes (Stegomyia) albopictus (Skuse, 1895) in Österreich 2012 (Diptera: Culicidae). Entomologische Zeitschrift. 2012;122(5):223-6.
32. Becker N, Geier M, Balczun C, Bradersen U, Huber K, Kiel E, et al. Repeated introduction of Aedes albopictus into Germany, July to October 2012. Parasitol Res. 2013 Apr;112(4):1787-90.
33. Bockova E, Kocisova A, Letkova V. First record of Aedes albopictus in Slovakia. Acta Parasitologica 2013;58(4):603-6.
34. Bonizzoni M, Gasperi G, Chen X, James AA. The invasive mosquito species Aedes albopictus: current knowledge and future perspectives. Trends Parasitol. 2013 Sep;29(9):460-8.
35. Oter K, Gunay F, Tuzer E, Linton YM, Bellini R, Alten B. First record of Stegomyia albopicta in Turkey determined by active ovitrap surveillance and DNA barcoding. Vector Borne Zoonotic Dis. 2013 Oct;13(10):753-61.
36. Carvalho RG, Lourenco-de-Oliveira R, Braga IA. Updating the geographical distribution and frequency of Aedes albopictus in Brazil with remarks regarding its range in the Americas. Memórias do Instituto Oswaldo Cruz. 2014 Sep;109(6):787-96.
37. Adeleke MA, Sam-Wobo SO, Garza-Hernandez JA, Oluwole AS, Mafiana CF, Reyes-Villanueva F, et al. Twenty-three years after the first record of Aedes albopictus in Nigeria: Its current distribution and potential epidemiological implications. African Entomology 2015;23(2):348-3.
38. Medlock JM, Hansford KM, Versteirt V, Cull B, Kampen H, Fontenille D, et al. An entomological review of invasive mosquitoes in Europe. Bulletin of Entomological Research. 2015 Dec;105(6):637-63.
39. Ngoagouni C, Kamgang B, Nakoune E, Paupy C, Kazanji M. Invasion of Aedes albopictus (Diptera: Culicidae) into central Africa: what consequences for emerging diseases? Parasit Vectors. 2015;8:191.
40. Prioteasa LF, Dinu S, Falcuta E, Ceianu CS. Established Population of the Invasive Mosquito Species Aedes albopictus in Romania, 2012-14. J Am Mosq Control Assoc. 2015 Jun;31(2):177-81.
41. Akiner MM, Demirci B, Babuadze G, Robert V, Schaffner F. Spread of the Invasive Mosquitoes Aedes aegypti and Aedes albopictus in the Black Sea Region Increases Risk of Chikungunya, Dengue, and Zika Outbreaks in Europe. PLoS Negl Trop Dis. 2016;10(4):e0004664.
42. Van den Hurk AF, Nicholson J, Beebe NW, Davis J, Muzari OM, Russell RC, et al. Ten years of the Tiger: Aedes albopictus presence in Australia since its discovery in the Torres Strait in 2005. One Health. 2016;2:19-24.
43. Scholte EJ, Dijkstra E, Blok H, De Vries A, Takken W, Hofhuis A, et al. Accidental importation of the mosquito Aedes albopictus into the Netherlands: a survey of mosquito distribution and the presence of dengue virus. Med Vet Entomol. 2008 Dec;22(4):352-44. Demeulemeester J, Deblauwe I, De Witte J, Jansen F, Hendy A, Madder M. First interception of Aedes (Stegomyia) albopictus in Lucky bamboo shipments in Belgium. Journal of the European Mosquito Control Association 2014;32:14-6.
45. Kampen H, Kronefeld M, Zielke D, Werner D. Further specimens of the Asian tiger mosquito Aedes albopictus (Diptera, Culicidae) trapped in southwest Germany. Parasitol Res. 2013 Feb;112(2):905-7.
46. Adhami J, Reiter P. Introduction and establishment of Aedes (Stegomyia) albopictus skuse (Diptera: Culicidae) in Albania. J Am Mosq Control Assoc. 1998 Sep;14(3):340-3.
47. Cornel AJ, Hunt RH. Aedes albopictus in Africa? First records of live specimens in imported tires in Cape Town. 1991 Mar;7(1):107-8.
48. Fontenille D, Toto JC. Aedes (Stegomyia) albopictus (Skuse), a potential new Dengue vector in southern Cameroon. Emerg Infect Dis. 2001;7(6):1066-7.
49. Sabatini A, Raineri V, Trovato G, Coluzzi M. [Aedes albopictus in Italy and possible diffusion of the species into the Mediterranean area]. Parassitologia. 1990 Dec;32(3):301-4.
50. Valerio L, Marini F, Bongiorno G, Facchinelli L, Pombi M, Caputo B, et al. Host-feeding patterns of Aedes albopictus (Diptera: Culicidae) in urban and rural contexts within Rome province, Italy. Vector Borne Zoonotic Dis. 2010 Apr;10(3):291-4.
51. Romi R, Di Luca M, Majori G. Current status of Aedes albopictus and Aedes atropalpus in Italy. J Am Mosq Control Assoc. 1999 Sep;15(3):425-7.
52. Wymann MN, Flacio E, Radczuweit S, Patocchi N, Luthy P. Asian tiger mosquito (Aedes albopictus) – a threat for Switzerland? Euro Surveill. 2008 Mar 6;13(10):8058.
53. Scholte EJ, Dijkstra E, Ruijs H, Jacobs F, Takken W, Hofhuis A, et al. The Asian tiger mosquito in the Netherlands: should we worry? . Proceed Section Exp Appl Entomol. 2007;18 131-6.
54. Scholte E, Den Hartog W, Dik M, Schoelitsz B, Brooks M, Schaffner F, et al. Introduction and control of three invasive mosquito species in the Netherlands, July-October 2010. Euro Surveill. 2010;15(45):19710.
55. Werner D, Kronefeld M, Schaffner F, Kampen H. Two invasive mosquito species, Aedes albopictus and Aedes japonicus japonicus, trapped in south-west Germany, July to August 2011. Euro Surveill. 2012;17(4):20067.
56. Werner D, Kampen H. Aedes albopictus breeding in southern Germany, 2014. Parasitol Res. 2015 Mar;114(3):831-4.
57. Kalan K, Kostanjšek R, Merdić E, Trilar T. A survey of Aedes albopictus (Diptera: Culicidae) distribution in Slovenia in 2007 and 2010. Natura Sloveniae. 2011;13(1):39-50.
58. Kalan K, Buzan VE, Ivović V. Distribution of two invasive mosquito species in Slovenia in 2013. Parasit Vectors. 2014;7(Suppl 1)(P9).
59. Mikov O, Nikolov G, Schaffner F, Mathis A, editors. First record and establishment of Aedes albopictus in Bulgaria. VBORNET-EMCA Joint Meeting ‘Invasive Mosquitoes and Public Health in the European Context’, ; 2013; Antwerp, Belgium, 28-29 November 2013.
60. Ganushkina LA, Tanygina E, Bezzhonova OV, Sergiev VP. [Detection of Aedes (Stegomyia) albopictus skus. Mosquitoes in the Russian Federation]. Meditsinskaya Parazitologya i Parazitarnye Bolezni. 2012 Jan-Mar(1):3-4.
61. Ganushkina LA, Bezzhonova OV, Patraman IV, Tanygina E, Sergiev VP. [Distribution of Aedes (stegomyia) aegypti l. and Aedes (stegomyia) albopictus skus. mosquitoes on the Black Sea coast of the Caucasus]. Med Parazitol I Parazitarnye Bolezni. 2013 Jan-Mar(1):45-6.
62. Caminade C, Medlock JM, Ducheyne E, McIntyre KM, Leach S, Baylis M, et al. Suitability of European climate for the Asian tiger mosquito Aedes albopictus: recent trends and future scenarios. J R Soc Interface. 2012 Oct 7;9(75):2708-17.
63. Fischer D, Thomas SM, Neteler M, Tjaden NB, Beierkuhnlein C. Climatic suitability of Aedes albopictus in Europe referring to climate change projections: comparison of mechanistic and correlative niche modelling approaches. Euro Surveill. 2014;19(6):20696.
64. Leisnham PT, Juliano SA. Impacts of climate, land use, and biological invasion on the ecology of immature Aedes mosquitoes: implications for La Crosse emergence. EcoHealth. 2012 Jun;9(2):217-28.
65. Roiz D, Neteler M, Castellani C, Arnoldi D, Rizzoli A. Climatic factors driving invasion of the tiger mosquito (Aedes albopictus) into new areas of Trentino, northern Italy. PloS one. 2011;6(4):e14800.
66. Wilkerson RC, Linton YM, Fonseca DM, Schultz TR, Price DC, Strickman DA. Making Mosquito Taxonomy Useful: A Stable Classification of Tribe Aedini that Balances Utility with Current Knowledge of Evolutionary Relationships. PloS one. 2015;10(7):e0133602.
67. Reinert JF, Harbach RE, Kitching IJ. Phylogeny and classification of Aedini (Diptera : Culicidae), based on morphological characters of all life stages. Zool J Linn Soc-Lond. 2004 Nov;142(3):289-368.
68. Medlock JM, Avenell D, Barrass I, Leach S. Analysis of the potential for survival and seasonal activity of Aedes albopictus (Diptera: Culicidae) in the United Kingdom. J Vector Ecol. 2006 Dec;31(2):292-304.
69. Thomas SM, Obermayr U, Fischer D, Kreyling J, Beierkuhnlein C. Low-temperature threshold for egg survival of a post-diapause and non-diapause European aedine strain, Aedes albopictus (Diptera: Culicidae). Parasit Vectors. 2012;5:100.
70. Romi R, Severini F, Toma L. Cold acclimation and overwintering of female Aedes albopictus in Roma. J Am Mosq Control Assoc. 2006 Mar;22(1):149-51.
71. Collantes F, Delgado JA, Alarcón-Elbal PM, Delacour S, J. L. First confirmed outdoor winter reproductive activity of Asian tiger mosquito (Aedes albopictus) in Europe. Anales de Biologia. 2014;36:71-6.
72. Roiz D, Rosa R, Arnoldi D, Rizzoli A. Effects of temperature and rainfall on the activity and dynamics of host-seeking Aedes albopictus females in northern Italy. Vector Borne Zoonotic Dis. 2010 Oct;10(8):811-6.
73. Giatropoulos A, Emmanouel N, Koliopoulos G, Michaelakis A. A study on distribution and seasonal abundance of Aedes albopictus (Diptera: Culicidae) population in Athens, Greece. J Med Entomol. 2012 Mar;49(2):262-9.
74. Turell MJ, Dohm DJ, Sardelis MR, Oguinn ML, Andreadis TG, Blow JA. An update on the potential of north American mosquitoes (Diptera: Culicidae) to transmit West Nile virus. J Med Entomol. 2005 Jan;42(1):57-62.
75. Delatte H, Dehecq JS, Thiria J, Domerg C, Paupy C, Fontenille D. Geographic distribution and developmental sites of Aedes albopictus (Diptera: Culicidae) during a Chikungunya epidemic event. Vector Borne Zoonotic Dis. 2008 Spring;8(1):25-34.
76. Juliano SA, Lounibos LP. Ecology of invasive mosquitoes: effects on resident species and on human health. Ecol Lett. 2005 May;8(5):558-74.
77. Genchi C, Rinaldi L, Mortarino M, Genchi M, Cringoli G. Climate and Dirofilaria infection in Europe. Vet Parasitol. 2009 Aug 26;163(4):286-92.
78. Vazeille M, Jeannin C, Martin E, Schaffner F, Failloux AB. Chikungunya: a risk for Mediterranean countries? Acta Trop. 2008 Feb;105(2):200-2.
79. Drago A, editor Education, information and public awareness in vector control. . 14th European Conference of the Society of Vector Ecology; 2003 September 3-6, 2003, ; Bellinzona, Switzerland.
80. Dieng H, Saifur RG, Hassan AA, Salmah MR, Boots M, Satho T, et al. Indoor-breeding of Aedes albopictus in northern peninsular Malaysia and its potential epidemiological implications. PloS one. 2010;5(7):e11790.
81. Qualls WA, Xue RD, Beier JC, Muller GC. Survivorship of adult Aedes albopictus (Diptera: Culicidae) feeding on indoor ornamental plants with no inflorescence. Parasitol Res. 2013 Jun;112(6):2313-8.
1. Paupy C, Delatte H, Bagny L, Corbel V, Fontenille D. Aedes albopictus, an arbovirus vector: from the darkness to the light. Microbes Infect. 2009 Dec;11(14-15):1177-85.
2. Invasive Species Specialist Group. Global Invasive Species Database – Aedes albopictus 2009. Available from: http://www.issg.org/database/species/ecology.asp?si=109&fr=1&sts=sss&lang=EN(link is external).
3. European Centre for Disease Prevention. Development of Aedes albopictus risk maps. Stockholm: European Centre for Disease Prevention and Control, 2009.
4. European Centre for Disease P, Control. The climatic suitability for dengue transmission in continental Europe. Stockholm: ECDC; 2012.
5. Gould EA, Higgs S. Impact of climate change and other factors on emerging arbovirus diseases. Trans R Soc Trop Med Hyg. 2009 Feb;103(2):109-21.
6. Kraemer MU, Sinka ME, Duda KA, Mylne AQ, Shearer FM, Barker CM, et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. Elife. 2015;4:e08347.
7. Benedict MQ, Levine RS, Hawley WA, Lounibos LP. Spread of the tiger: global risk of invasion by the mosquito Aedes albopictus. Vector Borne Zoonotic Dis. 2007 Spring;7(1):76-85.
8. Rezza G, Nicoletti L, Angelini R, Romi R, Finarelli AC, Panning M, et al. Infection with chikungunya virus in Italy: an outbreak in a temperate region. Lancet. 2007 Dec 1;370(9602):1840-6.
9. Grandadam M, Caro V, Plumet S, Thiberge JM, Souares Y, Failloux AB, et al. Chikungunya virus, southeastern France. Emerg Infect Dis. 2011 May;17(5):910-3.
10. Delisle E, Rousseau C, Broche B, Leparc-Goffart I, L’Ambert G, Cochet A, et al. Chikungunya outbreak in Montpellier, France, September to October 2014. Euro Surveill. 2015;20(17):21108.
11. La Ruche G, Souares Y, Armengaud A, Peloux-Petiot F, Delaunay P, Despres P, et al. First two autochthonous dengue virus infections in metropolitan France, September 2010. Euro Surveill. 2010 Sep 30;15(39):19676.
12. Marchand E, Prat C, Jeannin C, Lafont E, Bergmann T, Flusin O, et al. Autochthonous case of dengue in France, October 2013. Euro Surveill. 2013;18(50):20661.
13. Succo T, Leparc-Goffart I, Ferre JB, Roiz D, Broche B, Maquart M, et al. Autochthonous dengue outbreak in Nimes, South of France, July to September 2015. Euro Surveill. 2016 May 26;21(21):30240.
14. Gjenero-Margan I, Aleraj B, Krajcar D, Lesnikar V, Klobucar A, Pem-Novosel I, et al. Autochthonous dengue fever in Croatia, August-September 2010. Euro Surveill. 2011;16(9).
15. Schaffner F, Karch S. [First report of Aedes albopictus (Skuse, 1984) in metropolitan France]. C R Acad Sci III. 2000 Apr;323(4):373-5.
16. Madon MB, Mulla MS, Shaw MW, Kluh S, Hazelrigg JE. Introduction of Aedes albopictus (Skuse) in southern California and potential for its establishment. J Vector Ecol. 2002 Jun;27(1):149-54.
17. Schaffner F, Van Bortel W, Coosemans M. First record of Aedes (Stegomyia) albopictus in Belgium. J Am Mosq Control Assoc. 2004 Jun;20(2):201-3.
18. Eritja R, Escosa R, Lucientes J, Marques E, Roiz D, Ruiz S. Worldwide invasion of vector mosquitoes: present European distribution and challenges in Spain. Biol Invasions. 2005;7(1).
19. Aranda C, Eritja R, Roiz D. First record and establishment of the mosquito Aedes albopictus in Spain. Med Vet Entomol. 2006 Mar;20(1):150-2.
20. Contini C. Aedes albopictus in Sardinia: reappearance or widespread colonization? Parassitologia. 2007 Jun;49(1-2):33-5.
21. Haddad N, Harbach RE, Chamat S, Bouharoun-Tayoun H. Presence of Aedes albopictus in Lebanon and Syria. J Am Mosq Control Assoc. 2007 Jun;23(2):226-8.
22. Haddad N, Mousson L, Vazeille M, Chamat S, Tayeh J, Osta MA, et al. Aedes albopictus in Lebanon, a potential risk of arboviruses outbreak. BMC Infectious Diseases 2012;12:300.
23. Scholte EJ, Schaffner F. Waiting for the tiger: establishment and spread of the Aedes albopictus mosquito in Europe. In: Takken W, Knols BGJ, editors. Emerging pests and vector-borne diseases in Europe. 1. Wageningen, The Netherlands: Wageningen Academic Publishers, ; 2007. p. 241-60.
24. Buhagiar JA. A second record of Aedes (Stegomyia) albopictus (Diptera: Culicidae) in Malta. Eu Mosq Bull. 2009;27:65-7.
25. Diallo M, Laganier R, Nangouma A. First record of Ae. albopictus (Skuse 1894), in Central African Republic. Trop Med Int Health. 2010;15(10):1185-9.
26. Gatt P, Deeming JC, Schaffner F. First records of Aedes (Stegomyia) albopictus (Skuse) (Diptera: Culicidae) in Malta. Eu Mosq Bull. 2009;27 56-64.
27. Carrieri M, Albieri A, Angelini P, Baldacchini F, Venturelli C, Zeo SM, et al. Surveillance of the chikungunya vector Aedes albopictus (Skuse) in Emilia-Romagna (northern Italy): organizational and technical aspects of a large scale monitoring system. J Vector Ecol. 2011 Jun;36(1):108-16.
28. Izri A, Bitam I, Charrel RN. First entomological documentation of Aedes (Stegomyia) albopictus (Skuse, 1894) in Algeria. Clin Microbiol Infect. 2011;17(7):1116-8.
29. Fernandez Mdel C, Jean YS, Callaba CA, Lopez LS. The first report of Aedes (Stegomyia) albopictus in Haiti. Memórias do Instituto Oswaldo Cruz. 2012 Mar;107(2):279-81.
30. Sebesta O, Rudolf I, Betasova L, Pesko J, Hubalek Z. An invasive mosquito species Aedes albopictus found in the Czech Republic, 2012. Euro Surveill. 2012;17(43):20301.
31. Seidel B, Duh D, Nowotny M, Allerberger F. Erstnachweis der Stechmücken Aedes (Ochlerotatus) japonicus japonicus (Theobald, 1901) in Österreich und Slowenien in 2011 und für Aedes (Stegomyia) albopictus (Skuse, 1895) in Österreich 2012 (Diptera: Culicidae). Entomologische Zeitschrift. 2012;122(5):223-6.
32. Becker N, Geier M, Balczun C, Bradersen U, Huber K, Kiel E, et al. Repeated introduction of Aedes albopictus into Germany, July to October 2012. Parasitol Res. 2013 Apr;112(4):1787-90.
33. Bockova E, Kocisova A, Letkova V. First record of Aedes albopictus in Slovakia. Acta Parasitologica 2013;58(4):603-6.
34. Bonizzoni M, Gasperi G, Chen X, James AA. The invasive mosquito species Aedes albopictus: current knowledge and future perspectives. Trends Parasitol. 2013 Sep;29(9):460-8.
35. Oter K, Gunay F, Tuzer E, Linton YM, Bellini R, Alten B. First record of Stegomyia albopicta in Turkey determined by active ovitrap surveillance and DNA barcoding. Vector Borne Zoonotic Dis. 2013 Oct;13(10):753-61.
36. Carvalho RG, Lourenco-de-Oliveira R, Braga IA. Updating the geographical distribution and frequency of Aedes albopictus in Brazil with remarks regarding its range in the Americas. Memórias do Instituto Oswaldo Cruz. 2014 Sep;109(6):787-96.
37. Adeleke MA, Sam-Wobo SO, Garza-Hernandez JA, Oluwole AS, Mafiana CF, Reyes-Villanueva F, et al. Twenty-three years after the first record of Aedes albopictus in Nigeria: Its current distribution and potential epidemiological implications. African Entomology 2015;23(2):348-3.
38. Medlock JM, Hansford KM, Versteirt V, Cull B, Kampen H, Fontenille D, et al. An entomological review of invasive mosquitoes in Europe. Bulletin of Entomological Research. 2015 Dec;105(6):637-63.
39. Ngoagouni C, Kamgang B, Nakoune E, Paupy C, Kazanji M. Invasion of Aedes albopictus (Diptera: Culicidae) into central Africa: what consequences for emerging diseases? Parasit Vectors. 2015;8:191.
40. Prioteasa LF, Dinu S, Falcuta E, Ceianu CS. Established Population of the Invasive Mosquito Species Aedes albopictus in Romania, 2012-14. J Am Mosq Control Assoc. 2015 Jun;31(2):177-81.
41. Akiner MM, Demirci B, Babuadze G, Robert V, Schaffner F. Spread of the Invasive Mosquitoes Aedes aegypti and Aedes albopictus in the Black Sea Region Increases Risk of Chikungunya, Dengue, and Zika Outbreaks in Europe. PLoS Negl Trop Dis. 2016;10(4):e0004664.
42. Van den Hurk AF, Nicholson J, Beebe NW, Davis J, Muzari OM, Russell RC, et al. Ten years of the Tiger: Aedes albopictus presence in Australia since its discovery in the Torres Strait in 2005. One Health. 2016;2:19-24.
43. Scholte EJ, Dijkstra E, Blok H, De Vries A, Takken W, Hofhuis A, et al. Accidental importation of the mosquito Aedes albopictus into the Netherlands: a survey of mosquito distribution and the presence of dengue virus. Med Vet Entomol. 2008 Dec;22(4):352-8.
44. Demeulemeester J, Deblauwe I, De Witte J, Jansen F, Hendy A, Madder M. First interception of Aedes (Stegomyia) albopictus in Lucky bamboo shipments in Belgium. Journal of the European Mosquito Control Association 2014;32:14-6.
45. Kampen H, Kronefeld M, Zielke D, Werner D. Further specimens of the Asian tiger mosquito Aedes albopictus (Diptera, Culicidae) trapped in southwest Germany. Parasitol Res. 2013 Feb;112(2):905-7.
46. Adhami J, Reiter P. Introduction and establishment of Aedes (Stegomyia) albopictus skuse (Diptera: Culicidae) in Albania. J Am Mosq Control Assoc. 1998 Sep;14(3):340-3.
47. Cornel AJ, Hunt RH. Aedes albopictus in Africa? First records of live specimens in imported tires in Cape Town. 1991 Mar;7(1):107-8.
48. Fontenille D, Toto JC. Aedes (Stegomyia) albopictus (Skuse), a potential new Dengue vector in southern Cameroon. Emerg Infect Dis. 2001;7(6):1066-7.
49. Sabatini A, Raineri V, Trovato G, Coluzzi M. [Aedes albopictus in Italy and possible diffusion of the species into the Mediterranean area]. Parassitologia. 1990 Dec;32(3):301-4.
50. Valerio L, Marini F, Bongiorno G, Facchinelli L, Pombi M, Caputo B, et al. Host-feeding patterns of Aedes albopictus (Diptera: Culicidae) in urban and rural contexts within Rome province, Italy. Vector Borne Zoonotic Dis. 2010 Apr;10(3):291-4.
51. Romi R, Di Luca M, Majori G. Current status of Aedes albopictus and Aedes atropalpus in Italy. J Am Mosq Control Assoc. 1999 Sep;15(3):425-7.
52. Wymann MN, Flacio E, Radczuweit S, Patocchi N, Luthy P. Asian tiger mosquito (Aedes albopictus) – a threat for Switzerland? Euro Surveill. 2008 Mar 6;13(10):8058.
53. Scholte EJ, Dijkstra E, Ruijs H, Jacobs F, Takken W, Hofhuis A, et al. The Asian tiger mosquito in the Netherlands: should we worry? . Proceed Section Exp Appl Entomol. 2007;18 131-6.
54. Scholte E, Den Hartog W, Dik M, Schoelitsz B, Brooks M, Schaffner F, et al. Introduction and control of three invasive mosquito species in the Netherlands, July-October 2010. Euro Surveill. 2010;15(45):19710.
55. Werner D, Kronefeld M, Schaffner F, Kampen H. Two invasive mosquito species, Aedes albopictus and Aedes japonicus japonicus, trapped in south-west Germany, July to August 2011. Euro Surveill. 2012;17(4):20067.
56. Werner D, Kampen H. Aedes albopictus breeding in southern Germany, 2014. Parasitol Res. 2015 Mar;114(3):831-4.
57. Kalan K, Kostanjšek R, Merdić E, Trilar T. A survey of Aedes albopictus (Diptera: Culicidae) distribution in Slovenia in 2007 and 2010. Natura Sloveniae. 2011;13(1):39-50.
58. Kalan K, Buzan VE, Ivović V. Distribution of two invasive mosquito species in Slovenia in 2013. Parasit Vectors. 2014;7(Suppl 1)(P9).
59. Mikov O, Nikolov G, Schaffner F, Mathis A, editors. First record and establishment of Aedes albopictus in Bulgaria. VBORNET-EMCA Joint Meeting ‘Invasive Mosquitoes and Public Health in the European Context’, ; 2013; Antwerp, Belgium, 28-29 November 2013.
60. Ganushkina LA, Tanygina E, Bezzhonova OV, Sergiev VP. [Detection of Aedes (Stegomyia) albopictus skus. Mosquitoes in the Russian Federation]. Meditsinskaya Parazitologya i Parazitarnye Bolezni. 2012 Jan-Mar(1):3-4.
61. Ganushkina LA, Bezzhonova OV, Patraman IV, Tanygina E, Sergiev VP. [Distribution of Aedes (stegomyia) aegypti l. and Aedes (stegomyia) albopictus skus. mosquitoes on the Black Sea coast of the Caucasus]. Med Parazitol I Parazitarnye Bolezni. 2013 Jan-Mar(1):45-6.
62. Caminade C, Medlock JM, Ducheyne E, McIntyre KM, Leach S, Baylis M, et al. Suitability of European climate for the Asian tiger mosquito Aedes albopictus: recent trends and future scenarios. J R Soc Interface. 2012 Oct 7;9(75):2708-17.
63. Fischer D, Thomas SM, Neteler M, Tjaden NB, Beierkuhnlein C. Climatic suitability of Aedes albopictus in Europe referring to climate change projections: comparison of mechanistic and correlative niche modelling approaches. Euro Surveill. 2014;19(6):20696.
64. Leisnham PT, Juliano SA. Impacts of climate, land use, and biological invasion on the ecology of immature Aedes mosquitoes: implications for La Crosse emergence. EcoHealth. 2012 Jun;9(2):217-28.
65. Roiz D, Neteler M, Castellani C, Arnoldi D, Rizzoli A. Climatic factors driving invasion of the tiger mosquito (Aedes albopictus) into new areas of Trentino, northern Italy. PloS one. 2011;6(4):e14800.
66. Wilkerson RC, Linton YM, Fonseca DM, Schultz TR, Price DC, Strickman DA. Making Mosquito Taxonomy Useful: A Stable Classification of Tribe Aedini that Balances Utility with Current Knowledge of Evolutionary Relationships. PloS one. 2015;10(7):e0133602.
67. Reinert JF, Harbach RE, Kitching IJ. Phylogeny and classification of Aedini (Diptera : Culicidae), based on morphological characters of all life stages. Zool J Linn Soc-Lond. 2004 Nov;142(3):289-368.
68. Medlock JM, Avenell D, Barrass I, Leach S. Analysis of the potential for survival and seasonal activity of Aedes albopictus (Diptera: Culicidae) in the United Kingdom. J Vector Ecol. 2006 Dec;31(2):292-304.
69. Thomas SM, Obermayr U, Fischer D, Kreyling J, Beierkuhnlein C. Low-temperature threshold for egg survival of a post-diapause and non-diapause European aedine strain, Aedes albopictus (Diptera: Culicidae). Parasit Vectors. 2012;5:100.
70. Romi R, Severini F, Toma L. Cold acclimation and overwintering of female Aedes albopictus in Roma. J Am Mosq Control Assoc. 2006 Mar;22(1):149-51.
71. Collantes F, Delgado JA, Alarcón-Elbal PM, Delacour S, J. L. First confirmed outdoor winter reproductive activity of Asian tiger mosquito (Aedes albopictus) in Europe. Anales de Biologia. 2014;36:71-6.
72. Roiz D, Rosa R, Arnoldi D, Rizzoli A. Effects of temperature and rainfall on the activity and dynamics of host-seeking Aedes albopictus females in northern Italy. Vector Borne Zoonotic Dis. 2010 Oct;10(8):811-6.
73. Giatropoulos A, Emmanouel N, Koliopoulos G, Michaelakis A. A study on distribution and seasonal abundance of Aedes albopictus (Diptera: Culicidae) population in Athens, Greece. J Med Entomol. 2012 Mar;49(2):262-9.
74. Turell MJ, Dohm DJ, Sardelis MR, Oguinn ML, Andreadis TG, Blow JA. An update on the potential of north American mosquitoes (Diptera: Culicidae) to transmit West Nile virus. J Med Entomol. 2005 Jan;42(1):57-62.
75. Delatte H, Dehecq JS, Thiria J, Domerg C, Paupy C, Fontenille D. Geographic distribution and developmental sites of Aedes albopictus (Diptera: Culicidae) during a Chikungunya epidemic event. Vector Borne Zoonotic Dis. 2008 Spring;8(1):25-34.
76. Juliano SA, Lounibos LP. Ecology of invasive mosquitoes: effects on resident species and on human health. Ecol Lett. 2005 May;8(5):558-74.
77. Genchi C, Rinaldi L, Mortarino M, Genchi M, Cringoli G. Climate and Dirofilaria infection in Europe. Vet Parasitol. 2009 Aug 26;163(4):286-92.
78. Vazeille M, Jeannin C, Martin E, Schaffner F, Failloux AB. Chikungunya: a risk for Mediterranean countries? Acta Trop. 2008 Feb;105(2):200-2.
79. Drago A, editor Education, information and public awareness in vector control. . 14th European Conference of the Society of Vector Ecology; 2003 September 3-6, 2003, ; Bellinzona, Switzerland.
80. Dieng H, Saifur RG, Hassan AA, Salmah MR, Boots M, Satho T, et al. Indoor-breeding of Aedes albopictus in northern peninsular Malaysia and its potential epidemiological implications. PloS one. 2010;5(7):e11790.
81. Qualls WA, Xue RD, Beier JC, Muller GC. Survivorship of adult Aedes albopictus (Diptera: Culicidae) feeding on indoor ornamental plants with no inflorescence. Parasitol Res. 2013 Jun;112(6):2313-8.
82. Mitchell CJ. Geographic Spread of Aedes albopictus and Potential for Involvement in Arbovirus Cycles in the Mediterranean Basin. J Vector Ecol. 1995 Jun;20(1):44-58.
83. Straetemans M, Europe Ecgov-rrfcvti. Vector-related risk mapping of the introduction and establishment of Aedes albopictus in Europe. Euro Surveill. 2008 Feb 14;13(7):8040.
84. Severini F, Di Luca M, Toma L, Romi R. Aedes albopictus in Rome: results and perspectives after 10 years of monitoring. Parassitologia. 2008 Jun;50(1-2):121-3.
85. Effler PV, Pang L, Kitsutani P, Vorndam V, Nakata M, Ayers T, et al. Dengue fever, Hawaii, 2001-2002. Emerg Infect Dis. 2005 May;11(5):742-9.
86. Ramchurn SK, Moheeput K, Goorah SS. An analysis of a short-lived outbreak of dengue fever in Mauritius. Euro Surveill. 2009;14(34):19314.
87. Pampiglione S, Rivasi F, Angeli G, Boldorini R, Incensati RM, Pastormerlo M, et al. Dirofilariasis due to Dirofilaria repens in Italy, an emergent zoonosis: report of 60 new cases. Histopathology. 2001 Apr;38(4):344-54.
88. Gratz NG. Critical review of the vector status of Aedes albopictus. Med Vet Entomol. 2004 Sep;18(3):215-27.
89. Wong PS, Li MZ, Chong CS, Ng LC, Tan CH. Aedes (Stegomyia) albopictus (Skuse): a potential vector of Zika virus in Singapore. PLoS Negl Trop Dis. 2013;7(8):e2348.
90. Grard G, Caron M, Mombo IM, Nkoghe D, Mboui Ondo S, Jiolle D, et al. Zika virus in Gabon (Central Africa)–2007: a new threat from Aedes albopictus? PLoS Negl Trop Dis. 2014 Feb;8(2):e2681.
91. Schaffner F, Medlock JM, Van Bortel W. Public health significance of invasive mosquitoes in Europe. Clin Microbiol Infect. 2013 Apr 10;19:685-92.
92. Mitchell CJ, Niebylski ML, Smith GC, Karabatsos N, Martin D, Mutebi JP, et al. Isolation of eastern equine encephalitis virus from Aedes albopictus in Florida. Science. 1992 Jul 24;257(5069):526-7.
93. Turell MJ, Beaman JR, Neely GW. Experimental transmission of eastern equine encephalitis virus by strains of Aedes albopictus and A. taeniorhynchus (Diptera: Culicidae). J Med Entomol. 1994 Mar;31(2):287-90.
94. Gerhardt RR, Gottfried KL, Apperson CS, Davis BS, Erwin PC, Smith AB, et al. First isolation of La Crosse virus from naturally infected Aedes albopictus. Emerg Infect Dis. 2001 Sep-Oct;7(5):807-11.
95. Grimstad PR, Kobayashi JF, Zhang MB, Craig GB, Jr. Recently introduced Aedes albopictus in the United States: potential vector of La Crosse virus (Bunyaviridae: California serogroup). J Am Mosq Control Assoc. 1989 Sep;5(3):422-7.
96. Beaman JR, Turell MJ. Transmission of Venezuelan equine encephalomyelitis virus by strains of Aedes albopictus (Diptera: Culicidae) collected in North and South America. J Med Entomol. 1991 Jan;28(1):161-4.
97. Turell MJ, Beaman JR. Experimental transmission of Venezuelan equine encephalomyelitis virus by a strain of Aedes albopictus (Diptera: Culicidae) from New Orleans, Louisiana. J Med Entomol. 1992 Sep;29(5):802-5.
98. Holick J, Kyle A, Ferraro W, Delaney RR, Iwaseczko M. Discovery of Aedes albopictus infected with west nile virus in southeastern Pennsylvania. J Am Mosq Control Assoc. 2002 Jun;18(2):131.
99. Sardelis MR, Turell MJ, O’Guinn ML, Andre RG, Roberts DR. Vector competence of three North American strains of Aedes albopictus for West Nile virus. J Am Mosq Control Assoc. 2002 Dec;18(4):284-9.
100. Calzolari M, Bonilauri P, Bellini R, Albieri A, Defilippo F, Maioli G, et al. Evidence of simultaneous circulation of West Nile and Usutu viruses in mosquitoes sampled in Emilia-Romagna region (Italy) in 2009. PloS one. 2010;5(12):e14324.
101. Roiz D, Vazquez A, Rosso F, Arnoldi D, Girardi M, Cuevas L, et al. Detection of a new insect flavivirus and isolation of Aedes flavivirus in Northern Italy. Parasit Vectors. 2012;5:223.
102. Abramides GC, Roiz D, Guitart R, Quintana S, Guerrero I, Gimenez N. Effectiveness of a multiple intervention strategy for the control of the tiger mosquito (Aedes albopictus) in Spain. Trans R Soc Trop Med Hyg. 2011 May;105(5):281-8.
103. Worobey J, Fonseca DM, Espinosa C, Healy S, Gaugler R. Child outdoor physical activity is reduced by prevalence of the Asian tiger mosquito, Aedes albopictus. J Am Mosq Control Assoc. 2013 Mar;29(1):78-80.
104. Moutailler S, Barre H, Vazeille M, Failloux AB. Recently introduced Aedes albopictus in Corsica is competent to Chikungunya virus and in a lesser extent to dengue virus. Trop Med Int Health. 2009 Sep;14(9):1105-9.
105. Bonilauri P, Bellini R, Calzolari M, Angelini R, Venturi L, Fallacara F, et al. Chikungunya virus in Aedes albopictus, Italy. Emerg Infect Dis. 2008 May;14(5):852-4.
106. Thavara U, Tawatsin A, Pengsakul T, Bhakdeenuan P, Chanama S, Anantapreecha S, et al. Outbreak of Chikungunya Fever in Thailand and Virus Detection in Field Population of Vector Mosquitoes, Aedes Aegypti (L.) and Aedes Albopictus Skuse (Diptera: Culicidae). Se Asian J Trop Med. 2009 Sep;40(5):951-62.
107. de Lamballerie X, Leroy E, Charrel RN, Ttsetsarkin K, Higgs S, Gould EA. Chikungunya virus adapts to tiger mosquito via evolutionary convergence: a sign of things to come? Virol J. 2008;5:33.
108. Tilston N, Skelly C, Weinstein P. Pan-European Chikungunya surveillance: designing risk stratified surveillance zones. Int J Health Geogr. 2009;8:61.
109. Pierre V, Thiria J, Rachou E, Sissoko D, Lassalle C, Renault P. Epidémie de dengue 1 à la Réunion en 2004. . Journées de veille sanitaire, Paris; Paris2005. p. 64.
110. European Centre for Disease Prevention. Annual epidemiological report 2016. Dengue fever. Stockholm: European Centre for Disease Prevention; 2016. Available from: http://ecdc.europa.eu/en/healthtopics/dengue_fever/Pages/Annual-epidemiological-report-2016.aspx.
111. Rogers DJ, Suk JE, Semenza JC. Using global maps to predict the risk of dengue in Europe. Acta Trop. 2014 Jan;129:1-14.
112. Sousa CA, Clairouin M, Seixas G, Viveiros B, Novo MT, Silva AC, et al. Ongoing outbreak of dengue type 1 in the Autonomous Region of Madeira, Portugal: preliminary report. Euro Surveill. 2012;17(49):20333.
113. Chouin-Carneiro T, Vega-Rua A, Vazeille M, Yebakima A, Girod R, Goindin D, et al. Differential Susceptibilities of Aedes aegypti and Aedes albopictus from the Americas to Zika Virus. PLoS Negl Trop Dis. 2016 Mar;10(3):e0004543.
114. Di Luca M, Severini F, Toma L, Boccolini D, Romi R, Remoli ME, et al. Experimental studies of susceptibility of Italian Aedes albopictus to Zika virus. Euro Surveill. 2016 May 5;21(18):30223.
115. Jupille H, Seixas G, Mousson L, Sousa CA, Failloux AB. Zika Virus, a New Threat for Europe? PLoS Negl Trop Dis. 2016 Aug;10(8):e0004901.
116. Cancrini G, Frangipane di Regalbono A, Ricci I, Tessarin C, Gabrielli S, Pietrobelli M. Aedes albopictus is a natural vector of Dirofilaria immitis in Italy. Vet Parasitol. 2003 Dec 30;118(3-4):195-202.
117. Cancrini G, Romi R, Gabrielli S, Toma L, M DIP, Scaramozzino P. First finding of Dirofilaria repens in a natural population of Aedes albopictus. Med Vet Entomol. 2003 Dec;17(4):448-51.
118. Giangaspero A, Marangi M, Latrofa MS, Martinelli D, Traversa D, Otranto D, et al. Evidences of increasing risk of dirofilarioses in southern Italy. Parasitol Res. 2013 Mar;112(3):1357-61.
119. Poppert S, Hodapp M, Krueger A, Hegasy G, Niesen WD, Kern WV, et al. Dirofilaria repens infection and concomitant meningoencephalitis. Emerg Infect Dis. 2009 Nov;15(11):1844-6.
120. Weaver SC, Reisen WK. Present and future arboviral threats. Antiviral Res. 2010 Feb;85(2):328-45.
121. Seyler T, Grandesso F, Le Strat Y, Tarantola A, Depoortere E. Assessing the risk of importing dengue and chikungunya viruses to the European Union. Epidemics. 2009 Sep;1(3):175-84.
122. European Centre for Disease P, Control. Guidelines for the surveillance of invasive mosquitoes in Europe. Stockholm: ECDC; 2012.
123. Chan HH, Zairi J. Permethrin resistance in Aedes albopictus (Diptera: Culicidae) and associated fitness costs. J Med Entomol. 2013 Mar;50(2):362-70.
124. Tantely ML, Tortosa P, Alout H, Berticat C, Berthomieu A, Rutee A, et al. Insecticide resistance in Culex pipiens quinquefasciatus and Aedes albopictus mosquitoes from La Reunion Island. Insect Biochem Mol Biol. 2010 Apr;40(4):317-24.
125. Chen CD, Nazni WA, Lee HL, Norma-Rashid Y, Lardizabal ML, Sofian-Azirun M. Temephos resistance in field Aedes (Stegomyia) albopictus (Skuse) from Selangor, Malaysia. Trop Biomed. 2013 Jun;30(2):220-30.
126. Khan HA, Akram W, Shehzad K, Shaalan EA. First report of field evolved resistance to agrochemicals in dengue mosquito, Aedes albopictus (Diptera: Culicidae), from Pakistan. Parasit Vectors. 2011;4:146.
127. Baldacchino F, Caputo B, Chandre F, Drago A, della Torre A, Montarsi F, et al. Control methods against invasive Aedes mosquitoes in Europe: a review. Pest Management Science. 2015 Nov;71(11):1471-85.
Source: European Center Disease Control (ECDC)
Invasive mosquitoes colonising Europe – what can we do?
The Asian Tiger, Asian Bush and Yellow Fever mosquitos have made themselves at home in Europe throughout the last years, bringing with them some of the more exotic diseases, rarely seen in the EU before.
Are they a considerable threat to our health? What can we do to keep safe from the diseases they may spread?
Watch the animation:

The buzzing problem – invasive mosquitos colonising Europe
This content is hosted by a third party. By showing the external content you accept the terms and conditions of YouTube.
Source: European Center Disease Control (ECDC)
Dott. Alessio Brancaccio, tecnico ambientale Università degli Studi di L’Aquila, membro della Fondazione Michele Scarponi Onlus, ideologo e membro del movimento ambientalista Ultima Generazione A22 Network per contrastare il Riscaldamento Globale indotto artificialmente
